Selection of the hybridoma clone for a master seed
At the beginning of the hybridoma culture process, a clone must be generated and selected. The best clones are stable and good producers with high antibody production. Monoclonal antibodies are produced by immunizing an animal (usually a mouse) with a specific antigen. Subsequently B-lymphocyte plasma cells from the spleen of the immunized animal are removed. Since these B-cells are a primary cell line and have a limited lifespan in-vitro, they are fused with an immortal myeloma cell line, providing the ability to be sub-cultured indefinitely. The myeloma cell line can be derived from mice, other mammals, or human cells. Variations of standard monoclonal antibodies include bi-clonal, polyclonal, and chimeric versions e.g., up to 90% human, 10% mouse to improve acceptability for therapeutic applications.
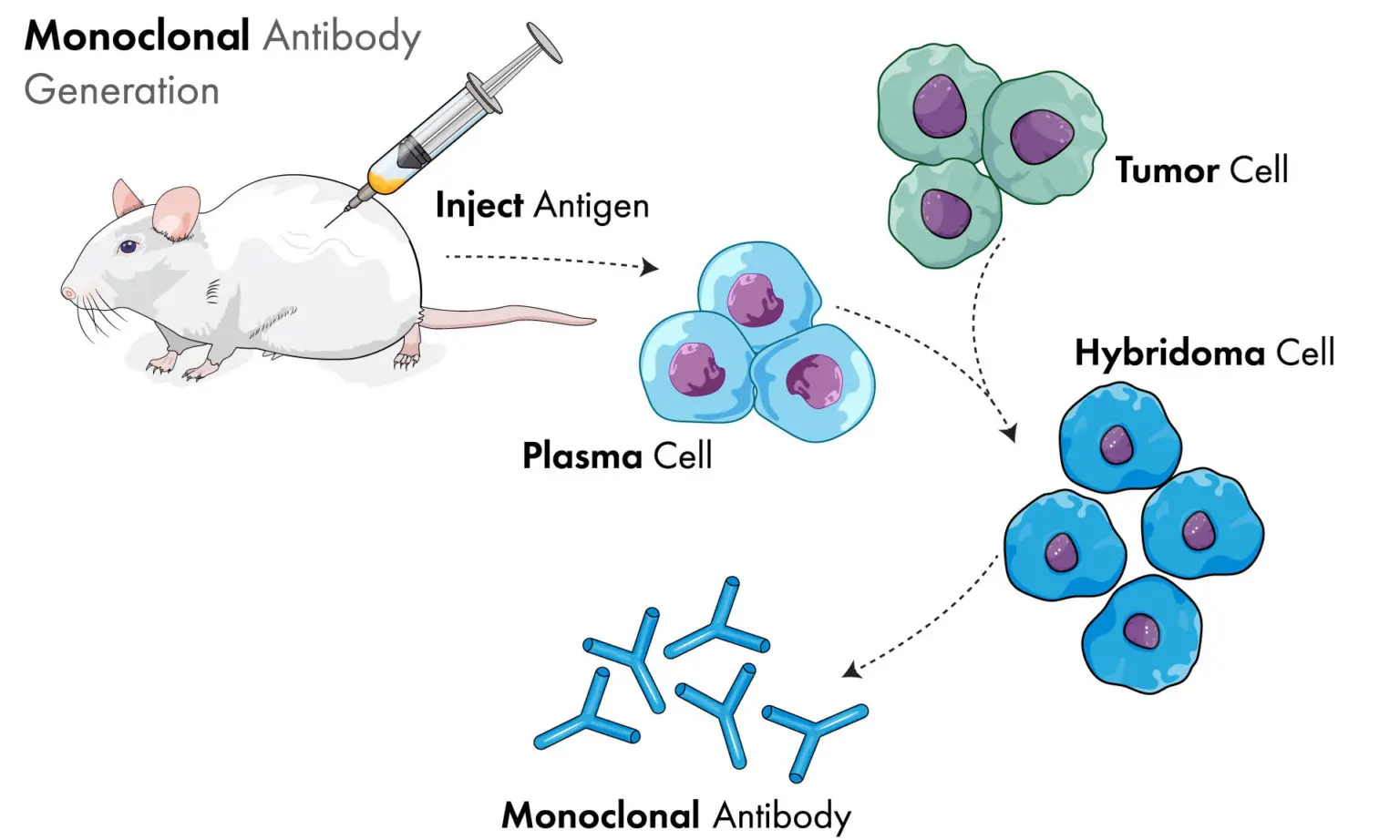
Hybridoma creation selection and expansion to create a master culture can be done at the bench-scale in static culture. The expansion phase of the most stable, productive clones is typically performed in serum-free media in T-flasks. Master cultures can be prepared from this material and stored in liquid nitrogen for revival to start the seed train of a bioprocess. The alternative would be an intensification step in a small bioreactor to produce cultures with a high cell density for direct inoculation into a pilot or production bioreactor. These master cultures will be periodically tested to ensure the specificity and productivity if the clone remains constant.
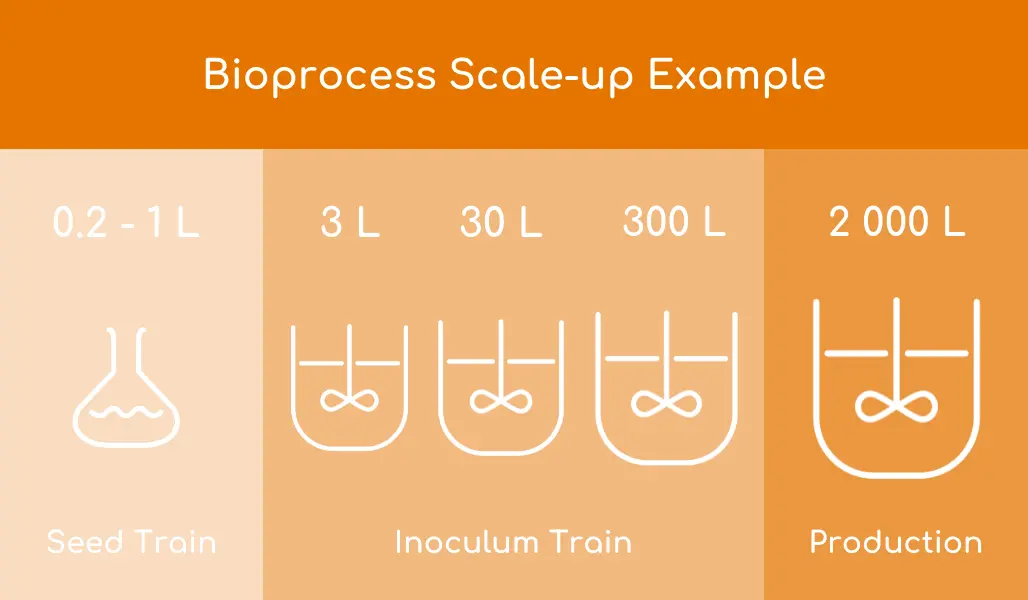
Seed train from vial to shake flasks
Seed train development is the gradual increase in cell density in a series of steps which cascades the cells produced from one step to the next largest. Each transfer can introduce its own potential problems in terms of contamination, failure to grow and loss of productivity. Minimizing the number of steps is clearly desirable. The latest developments in process intensification can remove many intermediate steps.
An incubator shaker with CO2 control designed specifically for cell culture is often used for early-stage seed development. Culture volumes can be as small as 20 mL at the start, moving in two or three steps to a litre or more. Multiple flasks are used at each stage to allow for potential problems and/or bulking of contents to provide the inoculum for the next stage. Of course, multi-deck systems can have several stages progressing in parallel.
A typical protocol for hybridoma cells [1] can look like the following:
| Temperature | 37°C |
| CO2 | 8% |
| Throw | 50 mm |
| Shaker speed | 120 min-1 |
| Humidity | 80% (not mentioned in the original publication, but commonly specified) |
Protein free media is available commercially and fill volumes for shake flasks should be around 20-25% (higher for some optimal growth flasks). To move from a 1 ml vial to 1 L of culture in a shake flask may require at least two intermediate steps in terms of flask capacity.
Scale-up to bench-top and pilot bioreactors
The bioreactor phase of scale-up typically involves bioreactors in a range from 10-50 liters. Often bench-scale bioreactors are running in a perfusion mode to produce high-density cultures making many of increasing volume steps unnecessary.
Typical specifications for a cell culture bioreactor include:
- A round bottom or dished vessel.
- Short aspect ratio e.g., 2:1.
- A marine impellor for low shear.
- Low gas flow rates and sparging with small bubbles.
- A gas mix system for 3 or 4 gasses, including CO2 for pH control.
- Slow-speed motor to provide gentle mixing.
- If applying continuous mode, a spin filter or similar is needed for continuous separation of supernatant from cells.
- Hybridoma culture in bioreactors can be cell line and conditioning dependent. These guidelines are, at best, an approximation to provide a starting point for bench-scale cultures [2]:
| Temperature | 37°C |
| pH | 7 |
| DO | 50% |
| Stirrer speed | 60 – 120 min-1 (a higher speed will reduce any cell leakage via a spin filter) |
| Gas mix | 2 gasses for air plus CO2 for pH control
|
Fed-batch operation can be achieved with daily feeding or, medium perfusion can be set at the rate of 0.5 – 1 working volumes per day. Batch, fed batch and perfusion cultures (using a spin filter or ATF filtration) can last for 7-14 days or longer after a batch phase of 3-4 days, depending on the process mode and clone selected.