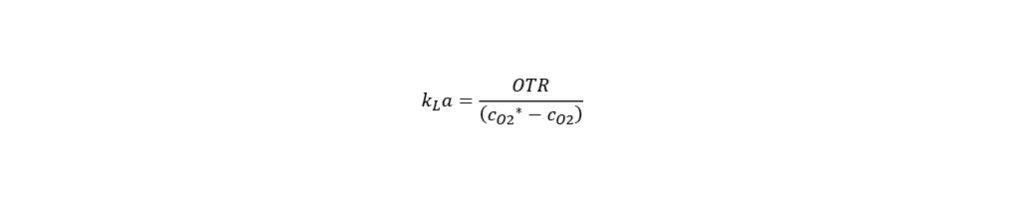
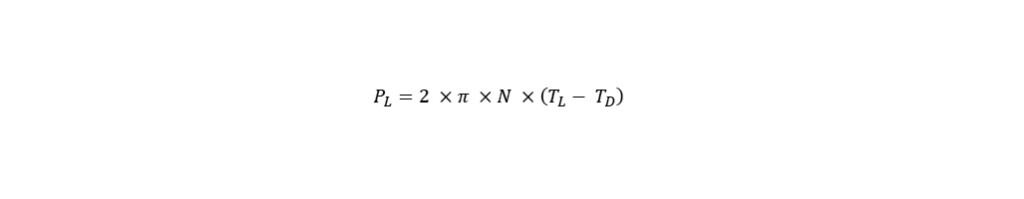Even today, scale-up is an erratic procedure since bioprocesses are not properly monitored and understood in detail. Two very important process relevant parameters are agitation and aeration. On the one hand, agitation is set to achieve homogenous mixing and oxygen transfer into the cells. Aeration, on the other hand, is regulated to supply the cells with sufficient amount of oxygen as well as to eliminate excess amount of CO2. Especially in large-scale bioreactors, excess CO2 concentration can pose a serious problem. Therefore, it is important to consider the aeration and agitation rates, position of the impeller and sparger, which will affect bubble size (Gray et al., 1996). The specific engineering parameters which are needed to successfully execute the scale-up process are discussed below at length (derived from DECHEMA, Society of Chemical Engineering and Biotechnology).
2. Power input
This engineering parameter is linked with hydrodynamic stress, which influences the growth of the cells and their productivity, particularly those of the more shear sensitive organisms. The most prominent technique to determine the power input is the torque-based measurement. This method is very easy to apply and is therefore the most frequently used. By using a torque sensor, the torque is measured on the stirrer shaft which then allows to determine power input. It must be pointed out that it is important to provide a vibration-free environment measurement. The formula below shows how the power input, P (W) can be calculated by determining the rotational agitator speed, N (min-1) and the torque in the liquid (N*m), as well as the torque in the empty vessel (N*m).

References:
Asnaghi, M., Smith, T., Martin, I., & Wendt, D. (2014). Bioreactors. https://doi.org/10.1016/B978-0-12-420145-3.00012-2
Bylund, F., Collet, E., Enfors, S. O., & Larsson, G. (1998). Substrate gradient formation in the large-scale bioreactor lowers cell yield and increases by-product formation. Bioprocess Engineering, 18(3), 171–180. https://doi.org/10.1007/s004490050427
Croughan, M. S., Hamel, J. F., & Wang, D. I. C. (1987). Hydrodynamic effects on animal cells grown in microcarrier cultures. Biotechnology and Bioengineering, 95(2), 295–305. https://doi.org/10.1002/bit.21158
Gray, D. R., Chen, S., Howarth, W., Inlow, D., & Maiorella, B. L. (1996). CO2 in large-scale and high-density CHO cell perfusion culture. Cytotechnology, 22(1–3), 65–78. https://doi.org/10.1007/BF00353925
Osman, J. J., Birch, J., & Varley, J. (2001). The response of GS-NS0 myeloma cells to pH shifts and pH perturbations. Biotechnology and Bioengineering, 75(1), 63–73. https://doi.org/10.1002/bit.1165
Xing, Z., Kenty, B. M., Li, Z. J., & Lee, S. S. (2009). Scale-up analysis for a CHO cell culture process in large-scale bioreactors. Biotechnology and Bioengineering, 103(4), 733–746. https://doi.org/10.1002/bit.22287