Consequently, two biological systems are used to produce recombinant proteins:
- to make the desired gene sequence available for translation – by using independently replicable plasmids as autonomous genetic elements
- to improve protein availability – by transfecting the plasmid into mammalian cells and expressing the corresponding gene sequence.
As one might expect, two different systems mean twice the work and money – especially in the regulated world of pharmaceutical manufacturing.
In contrast to research, transient transfections by plasmids are rarely used in industrial production. However, the production of stable cell lines in which the encoded gene sequence is incorporated into the genome is time-consuming. One of the more important reasons the mRNA approach has become the focus of research and development of advanced drugs, especially during the pandemic: The reduction of biological systems and through that time by direct use of the required genetic sequence. Traditional vaccine development and market launch take up to 10 years and longer. During the pandemic, this timeline needed to be shortened due to the urgency for vaccines.
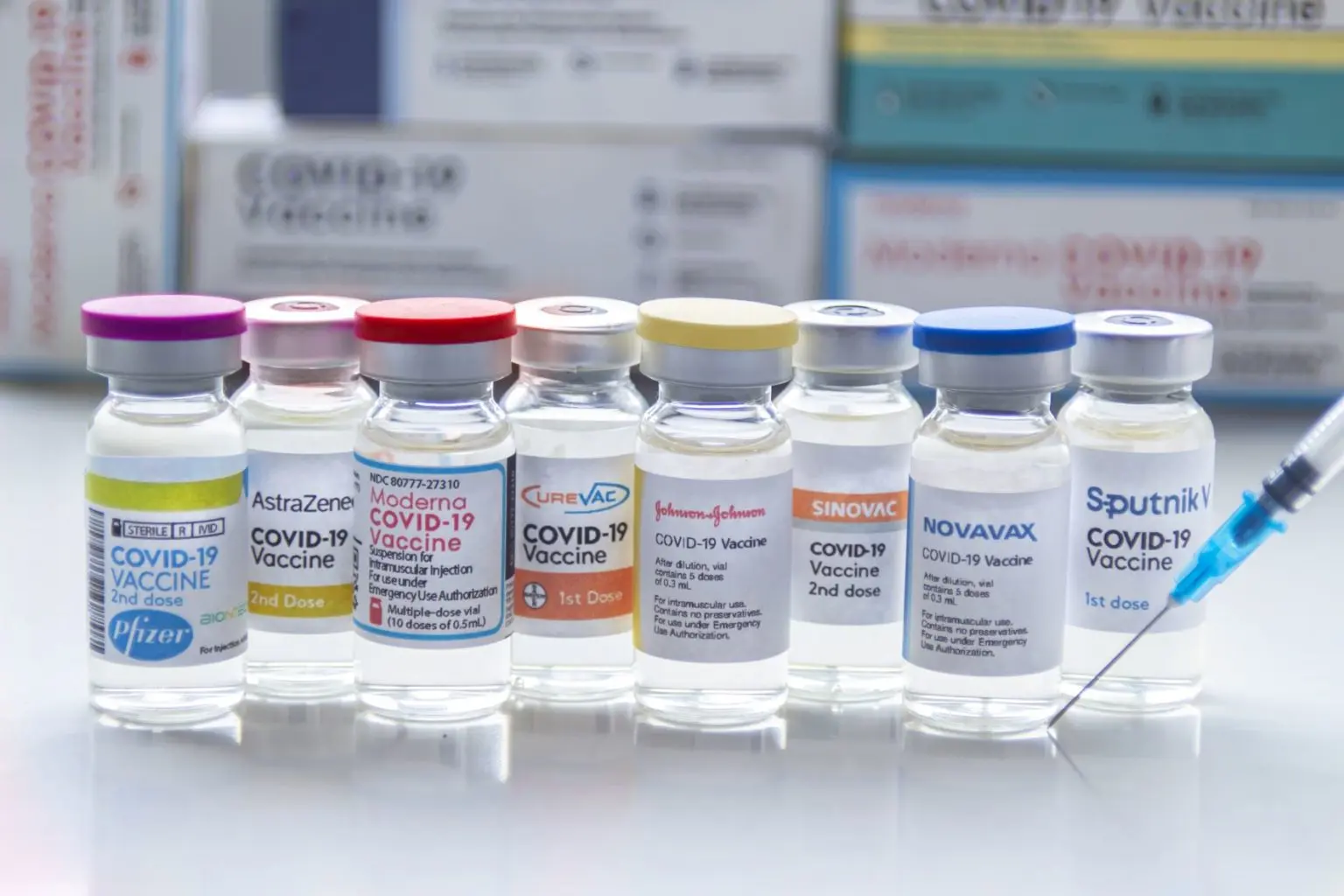
The mRNA vaccines currently available on the market are only suitable for the control of the COVID-19 pandemic. Reasons for the fast development and time to market were years of research on related viruses, faster manufacturing processes due to reduction of the second biological system (mammalian cells for proper protein post-translation) and accelerated regulatory processes as well as tremendous funding that allowed companies to conduct trials in parallel. Credit: Adobe Stock Images, 2022Although the potential of mRNA technology was already seen in 1978, when liposomes were used to transport mRNA into the cells of mice and humans in order to induce protein expression, the conventional wisdom among vaccine manufacturers was that mRNA was too unstable (prone to degradation) and too expensive to produce. The production of biologically active mRNA in the lab was seen primarily as a research tool to study gene function and activity.
In contrast, the idea of using mRNA technology in oncology as a therapeutic agent has been more favorably embraced. Several academic scientists and start-up companies investigated the potential use of mRNA to fight cancer. The expression of these proteins encoded by mRNA is expected to trigger an immune response to cancer cells.
mRNA Technology as therapeutic treatment
Development of mRNA technology for therapeutic use, Moderna Therapeutics & drug candidates in clinical trial
Researchers were already trying to turn mRNA into a drug platform in the 1990s but were repeatedly turned down by funding authorities. Problematic were potential immune responses from Toll-like receptors, which triggered massive inflammatory responses to the synthetic mRNA. The breakthrough to suppress the undesired immune reaction came with the conversion of chemical binding to the nucleotides of the mRNA. The therapeutic value of this and attention to its use as a therapeutic came in 2010 under the direction of Derrick Rossi, stem cell biologist and co-founder of Moderna Therapeutics, when skin cells were converted to muscle tissue using modified mRNA.
American 11-year-old pharmaceutical and biotechnology company, Moderna Therapeutics focuses on mRNA vaccines in particular and has long kept its science under the radar. Private investors were raised instead of selling their shares on the stock market, giving the company a reputation for secrecy as well as skepticism about its technology.
Nevertheless, with its initial public offering at the end of 2018, Moderna was able to raise more than $600 million to implement its ambitious plans to develop medicines.
As a result, the company has sparked an enthusiasm for therapeutic mRNA that is spreading to other start-ups and even big pharmaceutical companies. They clearly see the vision of revolutionizing the drug industry. What biotech companies like Amgen, Biogen and Genentech started in the 1980s with the development of protein therapies, mRNA technology can continue and replace.
As currently approved products on the market only include vaccines for COVID-19, further candidates in different fields are in clinical trial. Even Moderna has more than 20 candidates in its regulatory pipeline, including therapies for rare genetic diseases, cancer immunotherapies and vaccines against infectious diseases.
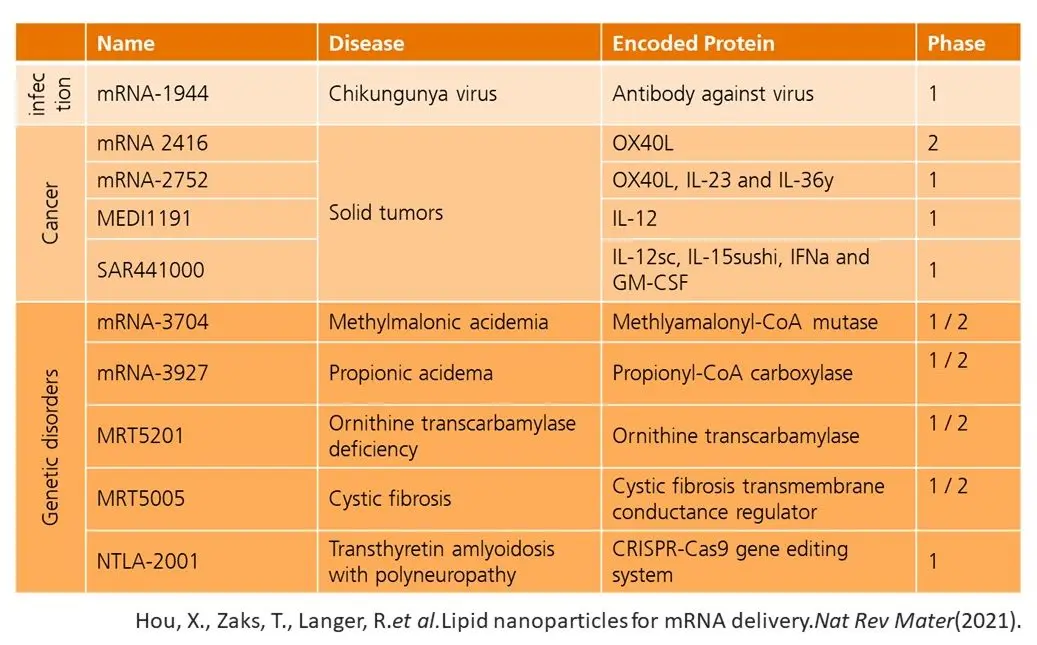
Through pharma partnerships with AstraZeneca, CureVac, Boehringer Ingelheim and Eli Lilly & Co, Moderna was not only able to draw attention to the field but leveraged mutual competencies to develop mRNA therapies. Successful use of mRNA therapeutics comes with solving key problems. Companies like Moderna must prove that:
- An immune response can be avoided
- Deliver therapy safely to the appropriate cells
- Translation mRNA delivers enough protein to have an effect
“Ultimately, you could use mRNA to express any protein and perhaps treat almost any disease. The possibilities are almost limitless.”
– Stephen Hoge, President Moderna Therapeutics
How to manufacture mRNA?
Compared to conventional vaccines, one of the most important advantages of mRNA is its relative simple production. Its production, in contrast to the classic in vivo synthesis, is done by an enzymatic in vitro transcription (IVT). This allows GMP facilities to standardize the process and act agilely when a new protein target comes into focus
To ensure certain quality characteristics, a series of manufacturing steps must be performed. Currently a number of combinations of steps are possible, which can be summarized as:
- genome sequencing target pathogen
- upstream processing
- template generation: production of pDNA or PCR amplification
- enzymatic generation of mRNA by IVT
- downstream processing: purification of the mRNA product and lipid nanoparticles (LNP) formulation as well as fill-to-finish steps
As we have already seen, the synthesis of mRNA starts from the corresponding DNA template. The template can be obtained by linearization of a purified plasmid or by amplification of the desired DNA by PCR. Thus, a plasmid can be the first step in the production of mRNA-based drugs.
With our expertise for the upstream process, we intend to take a closer look at the production of pDNA in our next article. To do so, our Innovation Manager, Damian von Blarer, in his double role as a scientific assistant at the FHNW, takes a look behind the scenes and explains how bioprocesses for the production of plasmids are optimized and carried out.