The 4 main types of stirred tank bioreactors by application are:
- Microbial bioreactors / Fermenters (Bacteria, Yeast, Fungi)
- Cell culture bioreactors (Mammalian cells, Insect cells)
- Single-use or disposable bioreactors (SUB’s used for cell culture)
- Bioreactors for special applications (SSF and Photobioreactors)
There is no coverage of non-STR systems in this overview, such as air lift, membrane bioreactors or fixed bed systems. They all have an important role for their specific applications but tend to be related to industries such as waste-water treatment.
1. Microbial bioreactors (fermenters)
A microbial stirred tank reactor will be equipped for rapid, high-shear mixing and good oxygen transfer. A tall height/diameter aspect ratio will be used to let bubbles of gas remain in contact with the culture for as long as possible. Multiple, flat-bladed Rushton turbine impellors break up gas bubbles with high shear forces to increase the surface area for gas exchange. Gas transfer rates are usually high, and foaming may become an issue. Microbial cultures have a long history in food production, industrial applications and the pharmaceuticals (white biotechnology).
Typical characteristics of microbial bioreactors / fermenters
- Tall height/diameter aspect ratio
- Multiple, flat-bladed Rushton turbine impellors
- High gas transfer rates
- Antifoam sensor and antifoam system
2. Cell culture bioreactors
Cell culture bioreactors will be equipped for reducing the chances of contamination over time, gentle mixing and use of gas mix for improved oxygen transfer. A broad marine impellor and slow-speed mixing protect cells from shear while ensuring good mixing. The coupling to the drive motor is usually magnetic. Oxygen transfer into the culture is enhanced using a low flow gas mixture with a variable concentration of pure oxygen. Products from these organisms are often for medical applications (red biotechnology).
Typical characteristics of cell culture bioreactors
- Gentle, low-speed mixing
- Broad marine impellor
- Low-flow gas mixture with precise pO2 control using air, O2 and N2
- CO2 instead of acid for pH control
3. Single use bioreactors
Single Use Bioreactors (SUB’s) are suitable for establishing a production train quickly when validation is a requirement. Looking at on-going costs, a single-use-bioreactor will save on cleaning and preparation times. However, it will need replacing after every culture. For validated processes, this makes a lot of sense. The advantage may not be so clear for other applications.
Typical characteristics of single-use, disposable bioreactors
- Pre-sterilized
- Less customization options
- Limited sensor options
- Leachable/extractable considerations
4. Bioreactors for special applications (SSF and Photobioreactors)
Special designs of bioreactor can be used for organisms which loosely fit into a fourth category. These may be algae, anaerobic bacteria or fungi growing on a solid substrate. Adaptation to the vessel design, geometry and mixing is necessary in these cases. The organisms are often associated with agriculture and biofuel applications (green biotechnology).
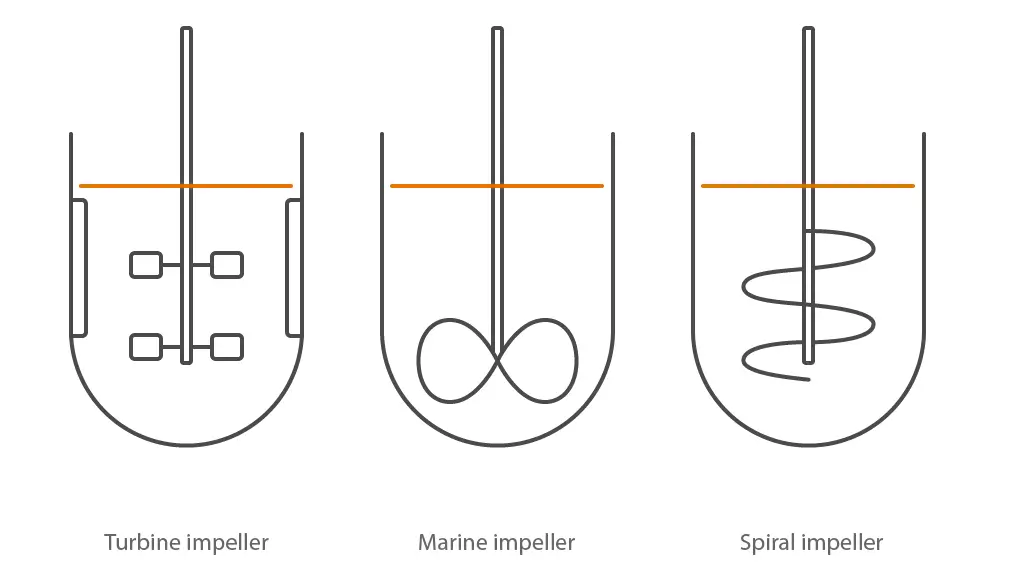
Illustration of the most common impeller designs used in stirred-tank bioreactors: Turbine impeller for microbial bioreactors; Marine impeller for cell culture bioreactors; Spiral impeller for solid substrates
Bioreactor types by size:
A big influence on what sort of bioreactor is needed will be the amount of culture per batch. There are three main choices for the typical stirred-tank bioreactor design:
- Small-scale, parallel bioreactors (under 1 L)
- Bench-scale bioreactors (1-10 L)
- Pilot-scale bioreactors (>10 L)
Deciding on scale should be based on the volume of batch culture required. A given size of bioreactor can be made more productive by the choice of feeding strategies. Options involved with gassing and mixing can increase the cell density per unit volume dramatically. A 2 L bioreactor could be a production volume for a high value therapeutic protein secreted from a specific clone.
Under 1 liter working volume
Screening applications where multiple, parallel bioreactor units are needed is a key feature at this scale. For working volumes under 100 ml, a highly parallel, disposable system makes sense in terms of handling and capacity for optimization. For working volumes of 100 – 1000 mL, a standard autoclavable glass vessel can be used. This allows adequate sample volumes to be removed and evaporation losses to be controlled per vessel. Multi-vessel systems are possible at this scale.
From 1 – 10 liters working volume
This is the classic scale for bench–top bioreactors. They allow more material to be produced for assay, downstream processing or limited production. The vessels used are typically made from glass and metal. They are sterilized by steam in an autoclave. An option for microbial bioreactors is a chemical sterilization in place (SIP). Single use vessels are an option for cell culture at this scale. They are pre-sterilized by irradiation following manufacture.
Above 10 litres working volume
The vessels are typically made from stainless steel and will be steam sterilized in situ (SIP). Often, they are also cleaned automatically in place (CIP). They are mostly free-standing. An option for single use bags as liners to simple steel vessels is possible between 50 – 1’000 litres. They are not common outside of pharmaceutical production.
Bioreactor types by feeding-strategy
How the requirements for growth are added and removed from a bioreactor of any type can greatly influence its operation. It allows a small vessel to produce a large volume or a very dense culture over time. The main categories are:
Batch
No liquid feed is added to the culture and nothing removed. This is a traditional approach and large vessel sizes allow commerical production
Fed-batch
Substrate is added over time according to the chosen feeding strategy (linear, step, dose or exponential). No culture is removed. This is common for high-density cultures. Some applications may need multiple feeds, with the change being used to trigger specific genes for switching from a growth phase to a production phase.
Continuous culture
Liquid substrate is both added and removed at a specific rate. Either substrate concentration or cell density is kept constant over a long time (days to months). This is highly productive but takes time and needs careful set-up. Perfusion is a variation on continuous culture most often applied to cell cultures. Culture supernatant is removed and fresh growth medium added. This needs a system to retain cells such as a spin filter or immolilization. The option is to let cells settle at fixed times and remove supernantant from the top of the culture. Fresh medium is added.
Without going into individual applications, there are already several criteria that set the structure for a bioreactor purchase. For example, is a large vessel for batch production better than a smaller unit run continuously?
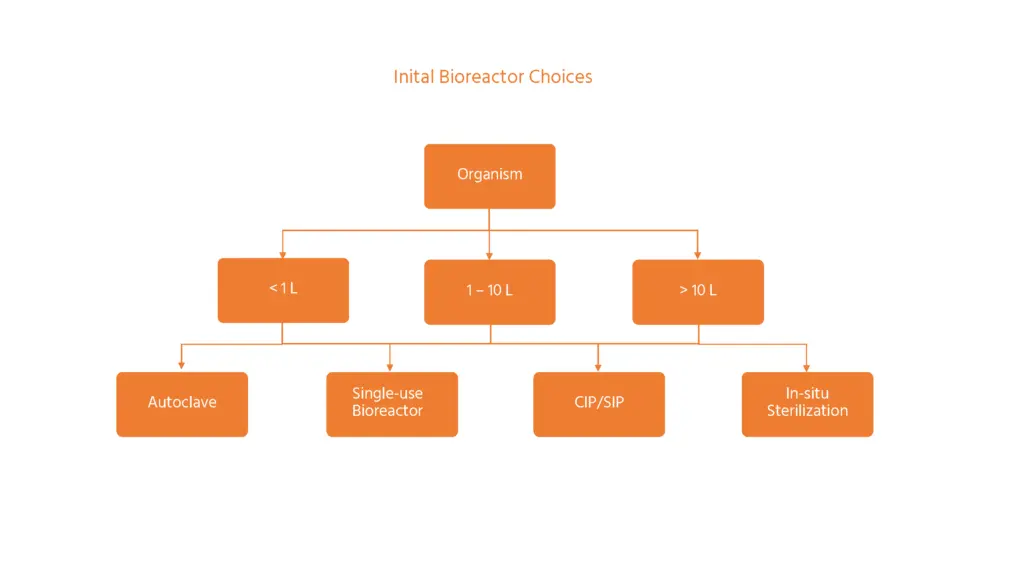
Initial bioreactor choices starting with the type of organism cultured, the required working volume of the vessel and the sterilization method.
Conclusion
The aim of this review is to list the choices available to anyone looking to move to a bioreactor from a more “universal” culture system such as shake flasks. It also provides some guidance according to organism and applications. The key points to take away are
- Know your organism and its requirements.
- Judge the scale you have to work at in terms of both volume and productivity needs.
- Decide if single-use systems can fully meet your needs, especially if the main use will be in a research environment.
- Look for specific adaptations to aid culture of your chosen microbe if the application is special.
- Put all this together into a list of requirements from which a User Requirements Specification (URS) can be created.