Step 1: Candidate generation – starting strong with smart tools
Every successful process begins with well-optimized candidates. Modern approaches rely on in silico optimization, secretion signal selection, and flexible expression vector designs. Machine learning enhances these steps, predicting how each cell line will perform from the start. Why it matters: This early integration ensures that product and process considerations are built in, reducing unnecessary steps later.
Step 2: Screening – testing, adjusting, and evaluating clones
Screening happens at a small scale to evaluate many potential clones in parallel. Using 96-well plates on automated incubator shakers, it is possible to control critical factors like mixing, temperature, CO2, and humidity. This creates a reproducible environment where results are consistent, even at high throughput.
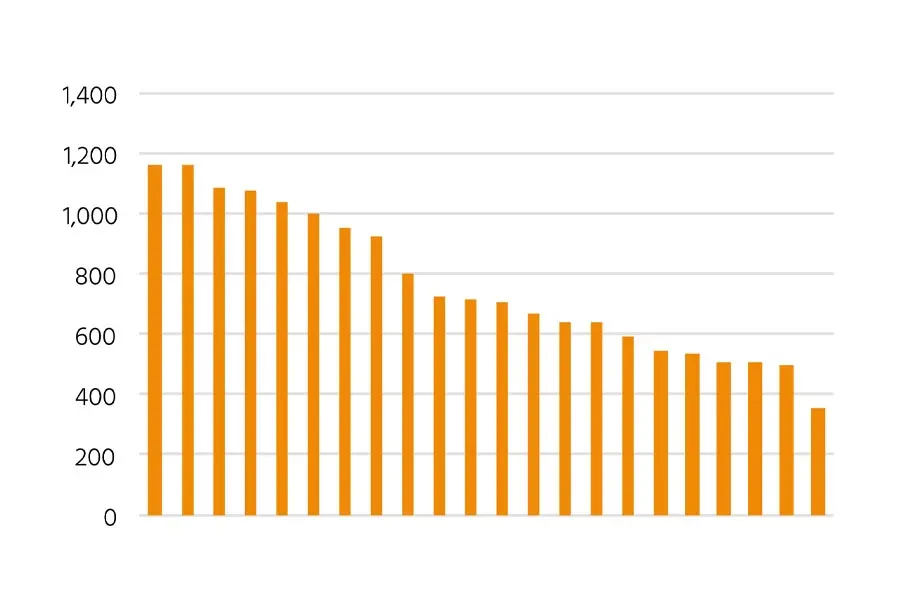
Figure 1: Comparison of productivity from the top 22 clones after initial screening. This highlights the variability in expression levels and the importance of robust screening methods.
Once promising clones are identified, they are further tested in 24-well deep plates to narrow down top candidates. Key tip: Efficient mixing is crucial for small-volume cultures. Standard incubators may not provide the agitation needed for 96-well plates, but a Multitron incubator shaker with a 3 mm shaking throw at 1,000 -1 significantly boosts cell titers and protein expression.
Step 3: Down selection – identifying top performers
After initial screening and selection, stable CHO cell lines are ranked based on productivity. The eight most promising clones are transferred to 125 mL shake flasks for further testing. Over a 14-day culture period, these clones are evaluated in the incubator shaker configured with a 25 mm orbit and agitation at 150 min-1. This step refines the selection, ensuring only the best-performing clones move forward.
Figure 2: Productivity data from the final eight selected clones in shake flask culture. This showcases the down selection process and how productivity varies even among the best candidates.
Step 4: Scaling up – from flasks to GMP production
After identifying the best-performing clones, the next step is scaling up. This can be done efficiently using incubator shakers rather than bioreactors. For example, Multitron incubator shakers support up to Growth flasksDid you know? With a triple stack Multitron incubator shaker, a researcher can cultivate 50 L of cell culture at a time, yielding up to 255 g of cell culture. This is often sufficient for early clinical studies, proving that bioreactors aren’t always necessary for small-scale GMP production.

Figure 3: Impact of shaking speed and orbital throw on CHO titers in 96-well plates. This illustrates why optimizing mixing parameters is crucial for efficient oxygen transfer and protein expression.
Tips for success
- Mixing matters: Uniform agitation is essential for reproducible results. At smaller scales, higher speeds (e.g., 800–1000 min-1) are needed to ensure adequate oxygen transfer.
- Control evaporation: Active humidification prevents losses in small volumes during long processes (10–14 days). This protects your culture’s integrity and ensures consistent yields.
- Automation saves time: Automated systems for screening and protein purification not only reduce variability but also increase throughput, allowing you to test more clones in less time.
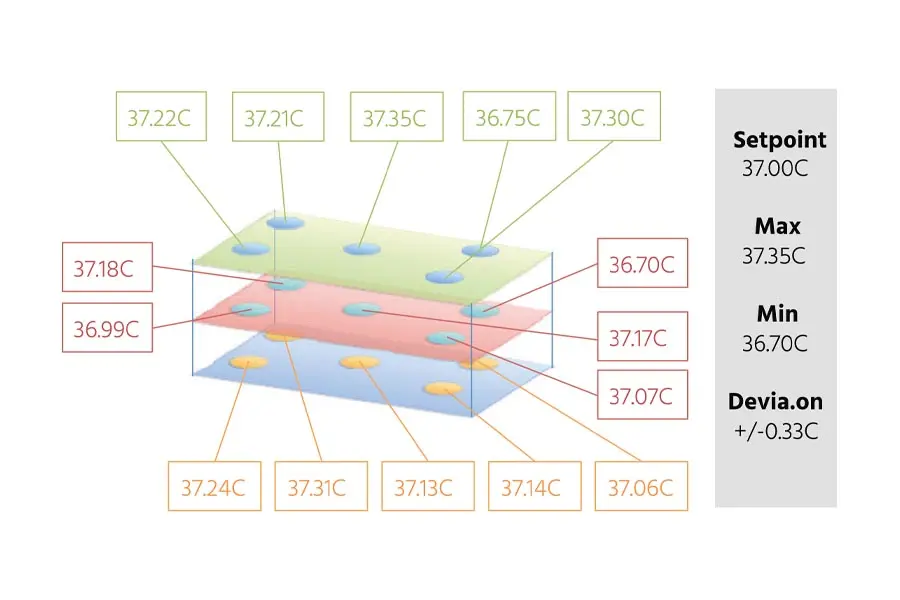
- Temperature uniformity is key: Maintaining consistent temperature across all culture vessels is critical to ensuring reliable growth and protein expression. Multitron shakers are designed to provide uniform heat distribution across every cabinet, preventing variations that could impact cell health and productivity.