Cell lines have much more complex needs than microbial organisms. This is especially true for control of most key parameters such as mixing, temperature, oxygenation, pH and foam. As the “aristocrats” of cultivation, cells are used to being maintained to a high standard. As the cells are part of larger structures, specific organs and processes they need a highly regulated environment. The role of the bioreactor is to emulate this as closely as possible.
The key features for successful CHO cell culture in the bioreactor include:
- Slow and gentle mixing
- High-end gassing system
- Foam prevention
- Accurate feeding
- Sterile sampling
These factors will not surprise experienced users, however, for researchers that are new to this method of cultivation it may provide help to get started. Even a baseline bioreactor which is simply run-in batch mode can produce results as good or better than shake flasks with larger volumes and will ensure increased level of process control.
Slow and gentle mixing relieves stress
The size of mammalian cells, plus their lack of a rigid cell wall, makes sensitivity to shear forces a critical factor in their survival. The environment in the bioreactor can be engineered to minimize shear forces and cell lines can also be adapted to tolerate higher levels of shear stress. This approach works for bioprocesses but application using primary cell lines won’t have that luxury.
The classical cell bioreactor solution includes a large, marine-type impellor.
This draws in culture from the edges and base of the vessel and circulates it vertically with minimal lateral shear stress. The precise mixing characteristics can be modelled using computerized flow dynamics (CFD).
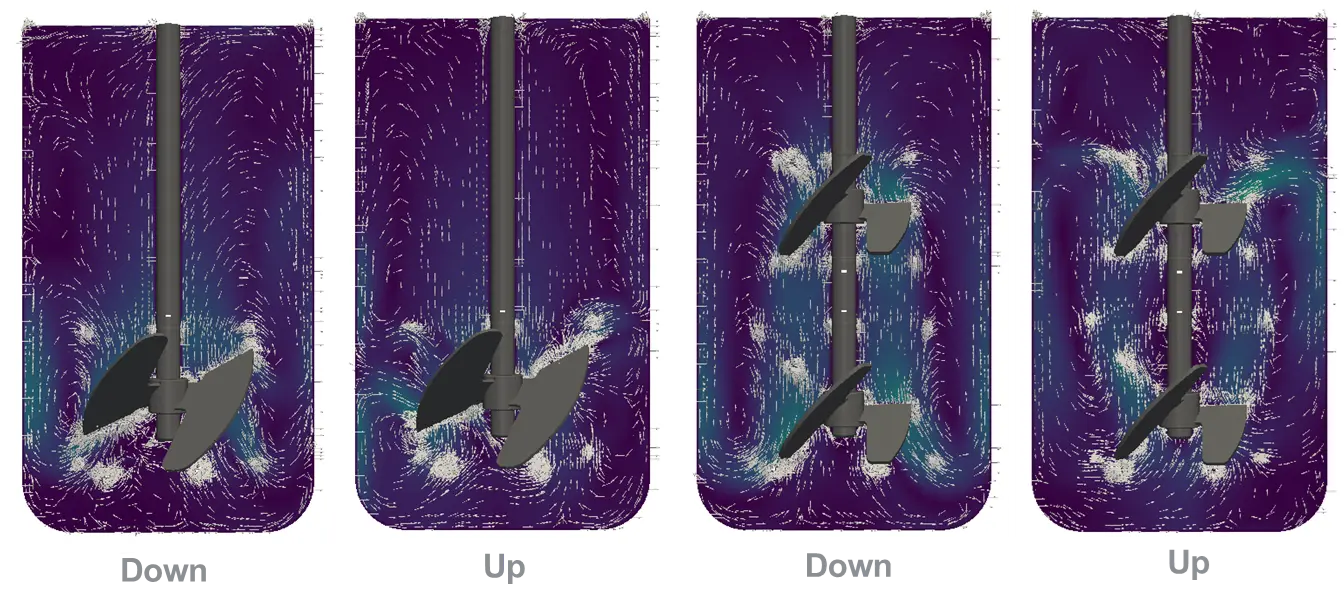
Minifors 2 Cell CFD simulation comparing one and two downwards as well upwards impellers. Results showed a lower maximum energy dissipation for one impeller, downwards. The Computational Fluid Dynamics (CFD) is a powerful engineering tool used to perform calculations, in which it is possible to simulate flow directions and power input.
Typically, stirrer speeds for cell cultures are much slower than for microbial cultures. Most cell lines are stirred at around 100 rpm. Some robust cell lines can survive higher speeds, but yield of biomass suffers. For a general overview on standard parameters for the most commonly used cell types have a look at our blog post: Typical Cultivation Parameters for Cell Cultures.
Getting the breathing right
In combination with the impeller, the sparger is responsible for the delivery of optimal sized gas bubbles. Therefore, it is critical that the sparger has holes of a specific size. How the gas reaches the culture, and its composition are separate topics. The method of entry into the vessel is easily dealt with as it is a binary choice, respectively a combination:
Into the headspace of the vessel
In this case, the gas transfer occurs at the gas/liquid interface. The advantages if this method include:
- No bubbles to cause foam
- a sight overpressure which actively suppresses foaming
- The accumulation of CO2 in the headspace caused by the metabolism of the cells is prevented
A larger surface area increases the gas transfer. Many cell culture vessels are now universal and have a taller aspect ratio (height to diameter 2.5:1). This favors an additional sparging of gas from the bottom of the vessel for maximum contact time with the medium.
Gassing via the sparger
This produces a steady stream of bubbles which can rise through the medium and exchange gas for as long as possible. Gassing via the sparger is more efficient but could cause foaming in cultures that have high levels of protein in the medium. If the foam suppression by the head space gassing is not sufficient an antifoaming agent might be added, as described below.
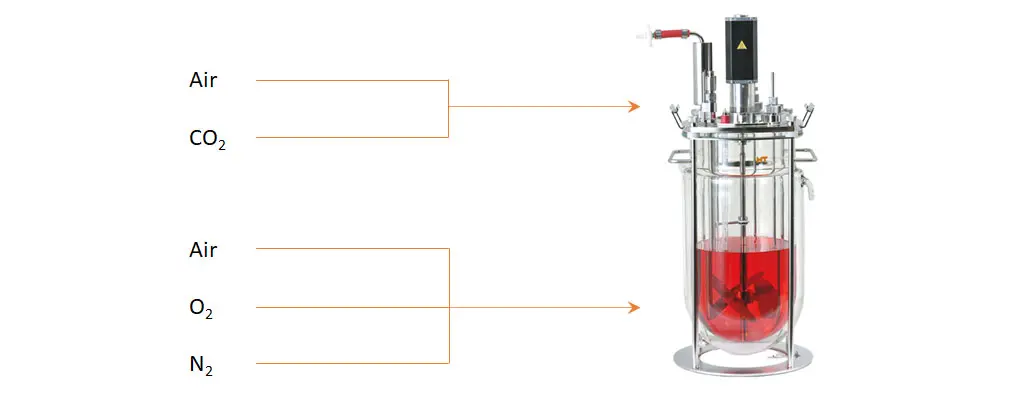
Example of a gassing strategy for CHO cells in a bioreactor. Air and CO2 are supplemented to the headspace. The Air/O2/N2 blend is supplied over the sparger.
We also need to consider how rapidly the gas is added. Mammalian cell cultures use oxygen and produce carbon dioxide at a slower rate than microbial cells. CHO cells have doubling times in the region of 20-24 hours. Lower gas flow rates up to 0.15 vvm (vessel volumes per minute) are gentler for sensitive cells and the risk of foam formation. The final part is the composition of the gas. Demand for oxygen increases with culture density and the slow mixing and low gas flow rates limit the ability to use oxygen naturally present in the air. On the other hand, too much oxygen at the start of the cultivation can be toxic to the cells.
Conclusion: The optimal gassing strategy for CHO Cell Cultures involves a combination of Headspace and Sparger gassing
The ability to change the composition of the gas entering the vessel by blending from 4 sources i.e., oxygen, nitrogen, air and carbon dioxide will solve the above issues. The air/N2/O2 mix can be automatically controlled by the pO2 parameter acting on individual mass flow controllers (MFCs). The CO2 is usually added to the mix based on the pH value. An additional mass flow controller (MFC) for the blended gas keeps the overall gas flow rate constant.
No head of foam wanted here
The presence of foam in a cell culture is not a bad sign. A light foam can be tolerated by most cell lines, but a dense foam can entrap cells and pull them apart. The presence of foam is due to proteins in the liquid medium and these can come from two sources:
- Protein-rich media. This is uncommon thanks to the many special formulations of CHO growth media available commercially.
- Protein secretion into the culture medium from the cells. This can be limited by using a perfusion technique to keep protein levels low by adding fresh medium and taking off protein-rich supernatant.
Adding a liquid antifoam reagent will reduce the foam but this can lead to problems if the product from the culture is to be used for therapeutic purposes. The antifoam reagent is a contaminant which may be difficult to remove during downstream processing. Moreover, too much antifoam agent can be harmful for the cells. However, foam can also be limited by keeping sparged gas flow rates low and/or using head space gassing.

Selection of different bioreactor vessels for CHO cells with a marine impeller.
Feeding them up – the ins and outs
The length of time cell cultures must be maintained can run from several days to a couple of weeks or longer. In that time, nutrients will be depleted, and toxic by-products can build up. Adding new substrate is an important way to prolong a cell cultivation. The accuracy and method will vary by application. By using a balance beneath the feed bottle, feed rates can be worked out by declining weight over time. Gravimetric feeding is the “gold standard” when it comes to cell cultivation. Add an automated system for e.g., dose feeding, and you have a flexible delivery solution integrated into the bioreactor.
A more advanced feeding strategy, that is often found in cell culture processes is perfusion. This type of continuous bioprocessing mode is based on either retaining the cells in the bioreactor or recycling the cells back to the bioreactor. Fresh medium is provided, and cell-free supernatant gets removed at the same rate. The advantages of this method are that it allows the maximum productivity and time for cleaning, sterilisation and handling of the vessel are all reduced.

Illustration of a CHO cell bioreactor vessel with an internal spin filter used for perfusion processes.
If you’d like to find out more about the different feeding strategies have a look at our blog-post: The Difference Between Batch, Fed-batch and Continuous Processes.
Sample with care
Extending a culture over many days will inevitably lead to an increased risk of contamination. It is prudent to check O rings, seals and tubing connections prior to starting each bioprocess. Simple single-use septa, feeding or inoculation needles should be used for inoculation. Once the bioprocess has begun, manual sampling from the vessel is one of the greatest risks of contamination. Sampling carries the highest risk as it is a repeated process through the same pipework each time. A microbial contaminant getting in will outgrow the cells and thrive in the nutrient-rich medium.
The best solution is to use a sampling system which reduces this possibility to a negligible amount. A good system to use is INFORS HT's Super Safe Sampler. The sampling device is made of single-use parts and sterilized by irradiation. Using a filter-protected line to draw a sample into a sterile syringe means the culture is protected during the sampling process. A blow-down system to remove any liquid culture in the sample line further reduces the chances of contamination. Tip: If phenol red is used in the growth medium as an indicator, an overnight change from red to yellow is a sure sign of microbial contamination.
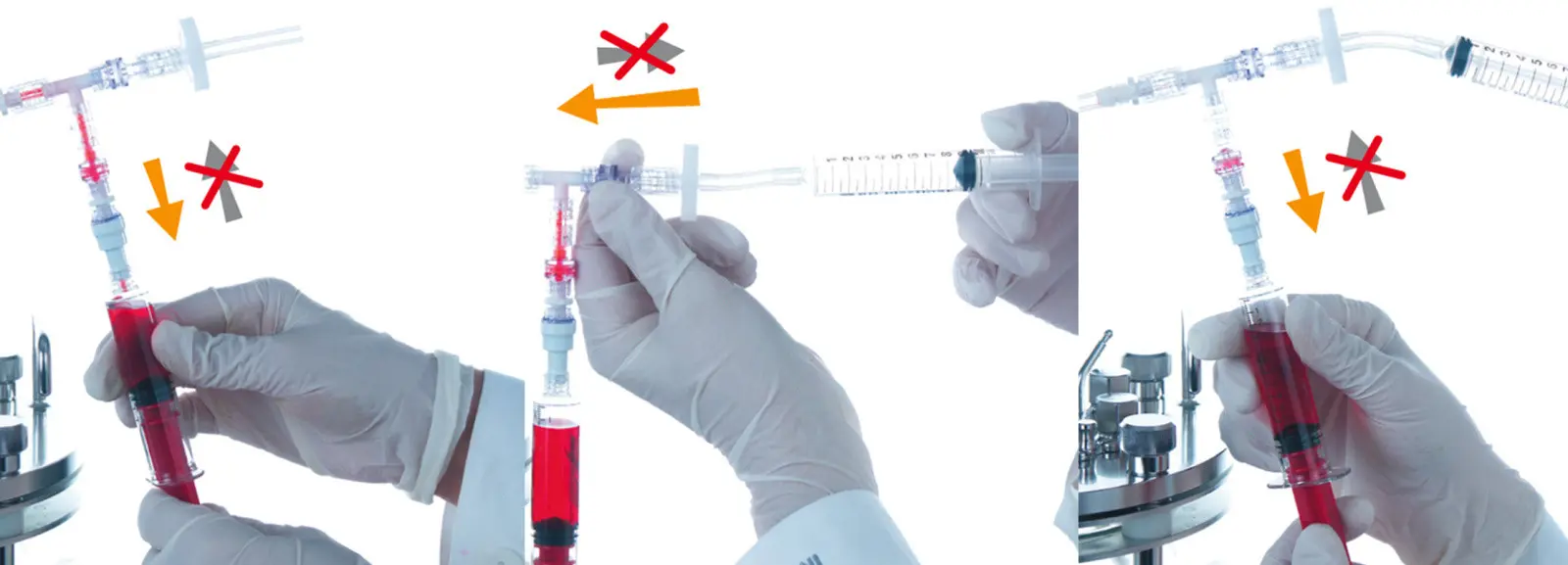
Sampling using the INFORS HT Super Safe Sampler.
For tasks such as estimating overall cell numbers or cell viability, an optical density probe or viable cell density sensor in the vessel can eliminate the need for sampling.