The move from shake flasks to a bioreactor needs to be made for compelling reasons. Generic arguments are fine but there must be a compelling case for your application. Grant-awarding bodies or senior managers must be convinced before a budget can be allocated. The arguments for why a bioreactor is needed include:
- There is a bottleneck in terms of enough material for chemical and biological assays.
- There is a gap in information about key parameters and processes.
- A multi-phase strategy is needed to produce the required material, and this cannot be done in shake flasks.
- It is a special application, such as solid-state fermentation, which would not be appropriate for shake flask cultures.
- The process has to be scaled-up using conventional stirred tank reactors (STR’s) where small-scale cultures can determine key scale-up parameters and generate usable data.
Key aspects about the potential benefits from moving to a bioreactor from shake flasks are discussed in more detail in this short article.
1. Higher yields
This is one of the main reasons for moving from shake flasks to a bioreactor – you can often get more in less time. This can be total biomass or yield of a specific product. A simple example is a direct comparison of the growth of E. coli (wild type e.g., K12) in shake flask versus a bioreactor.
Please note that the values used are a relative indication only and taken from several sources. In reality, several factors will influence your results, including:
- The strain (especially cloning, which may produce unwanted side effects such as shear sensitivity)
- Degree of optimization
- Substrate
- Operating conditions
Comparison of yields from shake flasks and bench-top bioreactors:
| Shake Flask, Batch overnight | OD600 ~ 4-6 |
| Bioreactor, Batch overnight | OD600 ~ 14-20 |
| Bioreactor, 1-day Fed-Batch | OD600 ~ 40 |
| Bioreactor, 2-day Fed-Batch | OD600 ~ 230 |
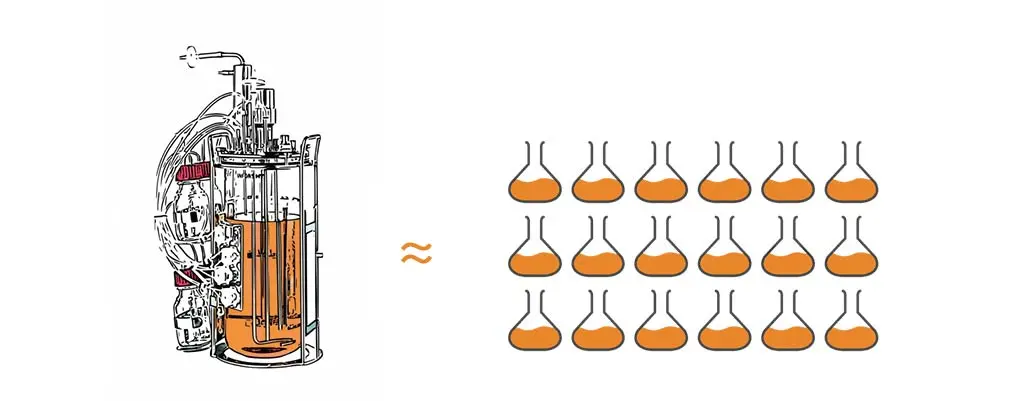
1 bench-top bioreactor with 3L working volume ~ a 3-deck incubator shaker with 18 5L flasks. This estimation is based on the following calculations: Cell Dry Weight CDW vs OD600 converts to ~0.36g/OD unit (coli, yeast). 1L OD 40 = 6.7L of OD 6.
2. Real-time bioprocess information
Listing the parameters for standard measurement and control of a bioreactor versus a shake flask shows the unsurprising result that more can be done with a bioreactor. Not all possibilities will be used in all cases, but the sheer range or possibilities can provide a good rationale for making the move. To keep the comparison as close as possible, a modern bench-scale bioreactor will be used:
Comparison of typical cultivation parameters in shake flasks and bioreactors:
Table: Comparison of bioreactor parameters versus incubator shaker parameters. (✓) relates to parameters which need additional equipment.
| Parameter | Shake Flask | Bioreactor |
| Temperature | ✓ | ✓ |
| Stirrer speed | ✓ | ✓ |
| Feed | (✓) | ✓ |
| pH | (✓) | ✓ |
| pO2 | (✓) | ✓ |
| Gas flow | (✓) | ✓ |
| Exit O2 and CO2 gas values | (✓) | ✓ |
| Gas mix | | (✓) |
| Biomass | (✓) | (✓) |
| Redox | | (✓) |
| Pressure | | (✓) |
| Individual gas control for blending | | (✓) |
| Conductivity | | (✓) |
| Turbidity | | (✓) |
While much more can be done with shake flasks using third-party equipment, cost, handling, and inter-operability issues all contribute to this being a niche solution for specific applications and circumstances.
3. Adaptability
One major difference between shake flasks and bioreactors, is the increased adaptability which comes from using a bioreactor. This is more than just the count of individual parameters which can be measured and controlled. The quality and range of measured values plus control capabilities are typically more restricted in shake flasks. Often, control of key parameters such as temperature is applied to all shake flasks at once. For many screening applications this may be unimportant but would be problematic for optimisation studies.
Examples of where adaptability plays a role include:
- The measured range may be more restricted for shake flask system e.g. pH range
- The opportunity to add at-line analyzers for additional information is restricted to bioreactors
- The opportunities to correct deviations in individual shake flasks may be limited e.g. if one flask needs more oxygen transfer, the shaker speed can only be changed for all the flasks
- Volumes for sampling for off-line analysis may be restricted from small shake flasks and require handling which is detrimental to overall growth e.g., sterility risks, changes in temperature and temporary lack of mixing.
- Adding a non-standard parameter is more difficult in terms of physical changes within the shaker and the use of additional software to be integrated with any over-arching bioprocess platform solution.
4. Better Control
This is a key element in the value of making the change to a bioreactor from shake flasks. This can be control of a single parameter or a complete process control using a phased strategy delivered from bioprocess software. Some useful control strategies can be achieved with shake flasks, but they are either limited in scope or require additional equipment.
Control strategies for shake flasks:
- Induction by temperature change
- Promotion of growth by changes in carbon dioxide concentration
- Extending culture duration by minimizing liquid loss due to evaporation
- Increase mixing and oxygenation with increasing biomass
- Induction and/or extending duration by change of substrate
For bioreactors, all the same possibilities exist but new ones extend the possibilities even further i.e.
- Multiple feeds in fed-batch or continuous modes
- Multi-parameter cascades for pO2 control
- Full two-sided pH control across a wide range of values
- Phased control by time, events and as a result of soft-sensor calculations
- Extended durations with continuous and perfusion methodologies

5. Scaling-up possibilities
Thanks to dramatic increase in yields over the last decade, small volumes of culture can contain high cell densities or product concentrations. This makes shake flask cultures a viable option for small scale production for applications such as early-stage clinical trials. However, where scale-up to commercial levels are anticipated, a small bioreactor can provide a good basis to establish the right criteria. This is due to simple physical similarity and the quality of information available i.e.:
- Stirred rank reactors (STR’s) are still the most common form for production-scale bioreactors and a bench-scale bioreactor can mimic this closely
- The key scale up criteria such as impellor tip speed and volumetric mass transfer are relevant to bioreactors but not directly to shake flasks
- Control strategies and optimizations from the small-scale bioreactor can often be applied at the larger scale with simple pro-rata changes
- Culture densities will be comparable and any limiting issues such as build-up of toxic products or shear sensitivity will be discovered at the bench scale.
- The same controller and instrumentation can often be used, with changes due to scale involving factors such as probe length and location
- Richer information can be extracted from the local controller, passed to bioprocess software, and used for analysis of scale-up criteria.

Summary
Bioreactors are sometimes seen as being too complex, too costly, and too time-consuming. It needs powerful arguments to make them the “go-to” choice. If this dilemma is familiar, the points below will help make the case:
- The improved mixing and aeration possible in a bioreactor allow for higher cell densities. This can be equated to faster results or more material for a given volume of culture.
- The degree of measurement and control possible will always be greater. Standard sensors and peripheral equipment can make a bioreactor far more adaptable to user requirements. This increases the quantity of data available for analysis and feedback into process development.
- The design of a stirred tank bioreactor means they are often more compact than rockers and incubator shakers of equivalent working volume. As bioreactors normally produce higher yields, a smaller system may be all that is needed.
- The range of automated feeding strategies is greater. For example, using bags or shake flasks for continuous culture is rare.
If you want maximum culture density, the best process information and scale-up/scale-down development, then a bench-scale bioreactor will be the right choice in almost all cases.
