The essential vector for genetic engineering: Plasmid DNA (pDNA)
Definition, function and importance of plasmids in biotechnological applications
If you need to produce large quantities of complex proteins (e.g., antibodies) quickly and cheaply, transient transfection is your way to go – starting with a plasmid. Plasmid DNA, also called plasmid or pDNA is a small extrachromosomal DNA molecule that can replicate independently. A popular host for plasmid production is the prokaryote E.coli.
As plasmids occur naturally in bacteria as well as in some eukaryotes, they often convey genetic advantages to the host, e.g., antibiotic resistance. When the host cell divides, the contained plasmids are copied as well if essential for survival. This is used in biotechnological applications to copy DNA fragments in large quantities and is also referred to as amplification of plasmids. Today, numerous plasmids are used for cloning, transferring and manipulating genes. With the possibility of inserting the gene of interest into these vectors, plasmids represent a key starting point for many genetic engineering techniques. From the development of recombinant proteins and viral vectors to advanced bio-therapeutics such as mRNA vaccines.
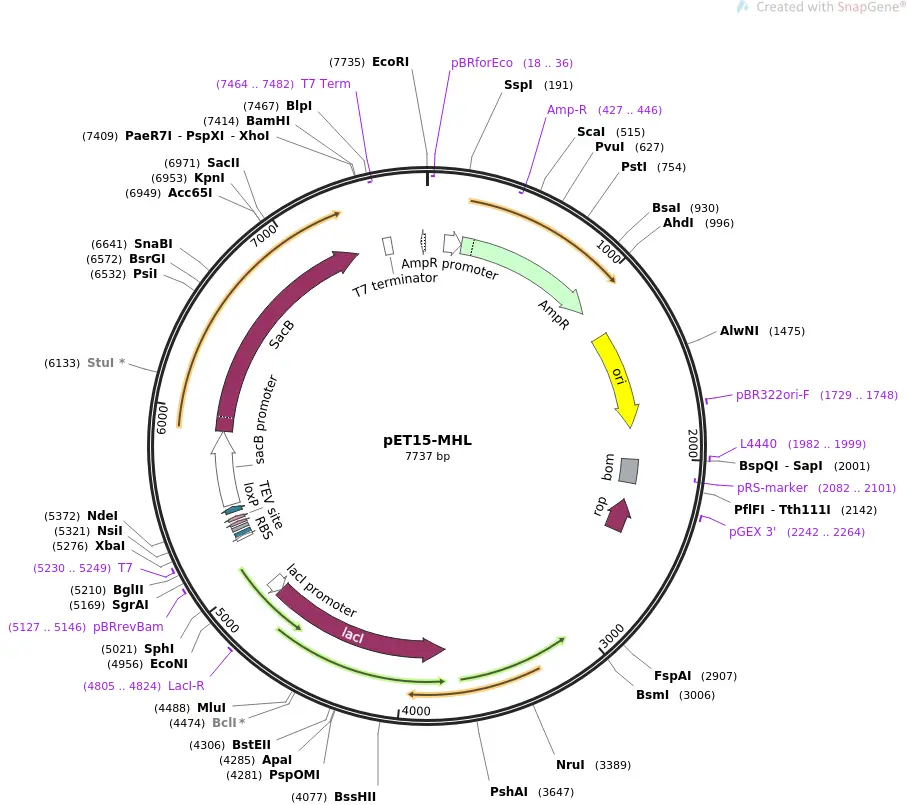
Various manufacturers offer established DNA vectors with numerous properties for a variety of uses such as genome engineering, visualization and tagging, purification, controlled expression or reporter plasmids etc. The following vector from Addgene can be induced (start expression) with lactose or IPTG and is well-liked for its high expressions of a recombinant protein. The 6x His epitope tag simplifies the purification process of a single protein.
In his position as a scientific assistant at the FHNW School of Life Sciences, Damian von Blarer conducts research at the pulse of time when it comes to biotechnological manufacturing processes. With the COVID-19 pandemic, the focus came to the development of novel bio-therapeutics and advanced testing methods.
“When we, like many other researchers, faced the challenge of producing specific parts of the spike protein as fast as possible, the easiest way was to use recombinant plasmids from E.coli.”
– Damian von Blarer, Researcher FHNW School of Life Sciences
The research group Bioprocess Technology faced the challenge of producing recombinant receptor binding domain (RBD) of the SARS-CoV-2 spike protein to contribute to the development of a serological assay for antibodies – Using Plasmids for this was his way to go.
How to use Plasmid DNA (pDNA): From Gene to Protein
Applied bioprocess – how to use plasmids for protein expression
Coronavirus belongs to the family of enveloped RNA viruses causing diseases in animals and humans. As obligate intracellular parasites, they depend on protein synthesis by host cells.
Consequently, fusion SARS-CoV-2 spike protein is also expressed and post-translated into a mammalian cell. Thus, it was clear to Damian and his colleagues, that a recombinant plasmid with the inserted gene sequence for the binding domain of the protein must be transfected into a mammalian cell. In fact, post-translational modifications of proteins after their synthesis allow temporal and spatial regulation of protein functions. Proper protein function is mandatory for further studies on the efficiency of the new developed Serological Antibody Rapid Test.
The transient transfection approach is ideal for the fast and cheap production of mammalian proteins and is often chosen in academia and biopharmaceutical research. Consequently, Damian has also chosen the following process for his task (illustration below).
Looking specifically at the process, it was possible to divide it into the following steps:
- Insertion gene of interest in empty pDNA
- Transformation (heat shock /electroporation to introduce pDNA into host bacteria / E.coli)
- Amplification pDNA: Cultivation in bioreactors to replicate bacterial host with inserted pDNA
- Quality Control (agarose gel electrophoresis & Optical Density 260 /280 ration)
- Purification plasmids
- Transfection into mammalian cells
- Protein expression in shaking flasks
- Quality Control (SDS-PAGE / Western Blot)
- Purification protein of interest
The amplification step represents a crucial initial stage for producing the targeted protein. In the next paragraph, the key questions to be addressed will provide a roadmap for the success of this crucial step.

For the production from the encoded gene to the desired protein, different biological (bacterial and mammalian cells) as well as equipment (bioreactor, shaker) were used. This will be used to produce the spike protein with appropriate modification. The steps are divided into translation, amplification, transfection and expression.
Amplification of pDNA: Questions for guiding successful bacterial cultivation
As we have seen from the previous section, plasmid amplification, in the most conventional cases, requires bacterial cultivation. The following steps should highlight important issues regarding the basis and guidelines.
#1 Strategy
- What is your goal & product of interest?
- Which microorganism is suitable?
- Has the proper growth media been selected?
- What are the Critical Process Parameters (CPP such as pO2, c-source, pH) & how can you control them (e.g. feeding additives such as gas, c-source, acid, base)?
- What are the Critical Quality Attribute (CQA such as RNA / DNA ration, iso-form plasmid e.g. supercoiled, circle, linear) derived from the CPP & how can you measure them?
- How many & what kind of samples do you need – are the labels for it ready?
- What is the schedule for the bioprocess? Are samplings scheduled at unusual times (e.g. night)?
#2 Bioreactor Set-up
- Which additives does your bioprocess need to ensure the control of defined CPP?
- How can you measure your CQA – what kind of Sensors do you need?
- How do you inoculate (e.g. needle or push-valve)?
- Do you have the appropriate peripherals & ports for inoculation?
#3 Autoclave
- Where are the sterile barriers – how can you ensure sterility?
- Do you have heat-sensitive components in the media (e.g. vitamins, amino acids)?
- Which autoclave program is used?

Autoclaving often is a critical point with no / or overly complicated return: after this step, it is important to pay attention to a neat sterile technique – the better the preparation beforehand, the easier and safer the sterile work afterwards.
#4 Inoculum
- What is your initial biomass (x0) in your bioreactor – how much biomass (e.g., OD, g/L) do you need?
- Is the culture in the exponential growth phase?
- When do you have to seed the pre-culture to reach the desired cell density in the flask so that there is enough biomass for inoculation?
#5 “good to go”
- Was the medium in the bioreactor complemented and buffered after autoclaving?
- Are all correcting agents and connections to the sensors connected?
- Have you left the system overnight to check sterility? – was the sterile hold successful?

Design your fermentation schedule in such a way that you can use periods of free time, e.g. for sterile control of the system, by leaving the complemented medium overnight.
#6 Inoculation
- How do you get the pre-culture into the bioreactor?
- Do you need additional pumps or sterile bottles?
- Do you have everything ready to quickly and carefully pierce the membrane?
- Was the pO2 calibrated before inoculation?
#7 Monitoring & Sampling
- Have you started the bioprocess software for monitoring and control?
- Are all signals being transmitted?
- Is your sampling schedule & labels set?
- Does your CPP measurement work?
Correct labelling of your samples is important for the preparation, control and traceability of your bioprocess – prepare the labels early enough so that you have them handy during the process.
#8 Harvest & Cleaning
- Do you have the necessary connections, conveyors, and containers for a sterile harvest?
- How can you re-sterilize the remaining culture and make it harmless to the environment?
- Where can you safely dispose of them?
- Have you checked all hoses, caps and filters for reuse during cleaning?
- Did you prepare the bioreactor vessel to autoclave?
Sources:
Brosi L, Kübler E, Weston A, Romann P, Panikulam S, Dirscherl L, Gerspach M, Giegelmann C, Dolce D, Überschlag ME, Melone A, Bantleon FI, Villiger TK, Gerhold CB. Development of a Unique Rapid Test to Detect Anti-bodies Directed Against an Extended RBD of SARS-CoV-2 Spike Protein. Chimia (Aarau). 2021 May 28;75(5):446-452. doi: 10.2533/chimia.2021.446