What are the benefits of multiple ports?
Ports on the top plate of a bioreactor allow you to
- Add a new sensor
- Cell-free sampling to feed an at-line analyzer
- Increase the capacity for different feed strategies, e.g. change from fed-batch to continuous operation
- Remove the need for needles when inoculating
- Improve handling by being able to re-position sensors, pipes etc. on the top plate
How to increase port capabilities
Tip #1: Optimize use of existing connectors
Readily available single-use connectors make most of the options easy to implement. The use of connectors allows aseptic removal and addition of lines during the culture. A little pre-planning and stocking up on basic items is all that is required. The starting place is knowing how many ports of which sizes are fitted and how many inlets, outlets, sensors plus fittings you need
The location of the ports also plays a part, as it may be difficult to connect some items close to each other if they “overhang” the port. An exit gas cooler is a prime example where the diameter of the main body of the unit is wider than the port diameter. The same can apply to the drive motor, depending how far above the vessel top plate it sits. A related point is any thermal blocks, a heat jacket or any external part which can limit access.
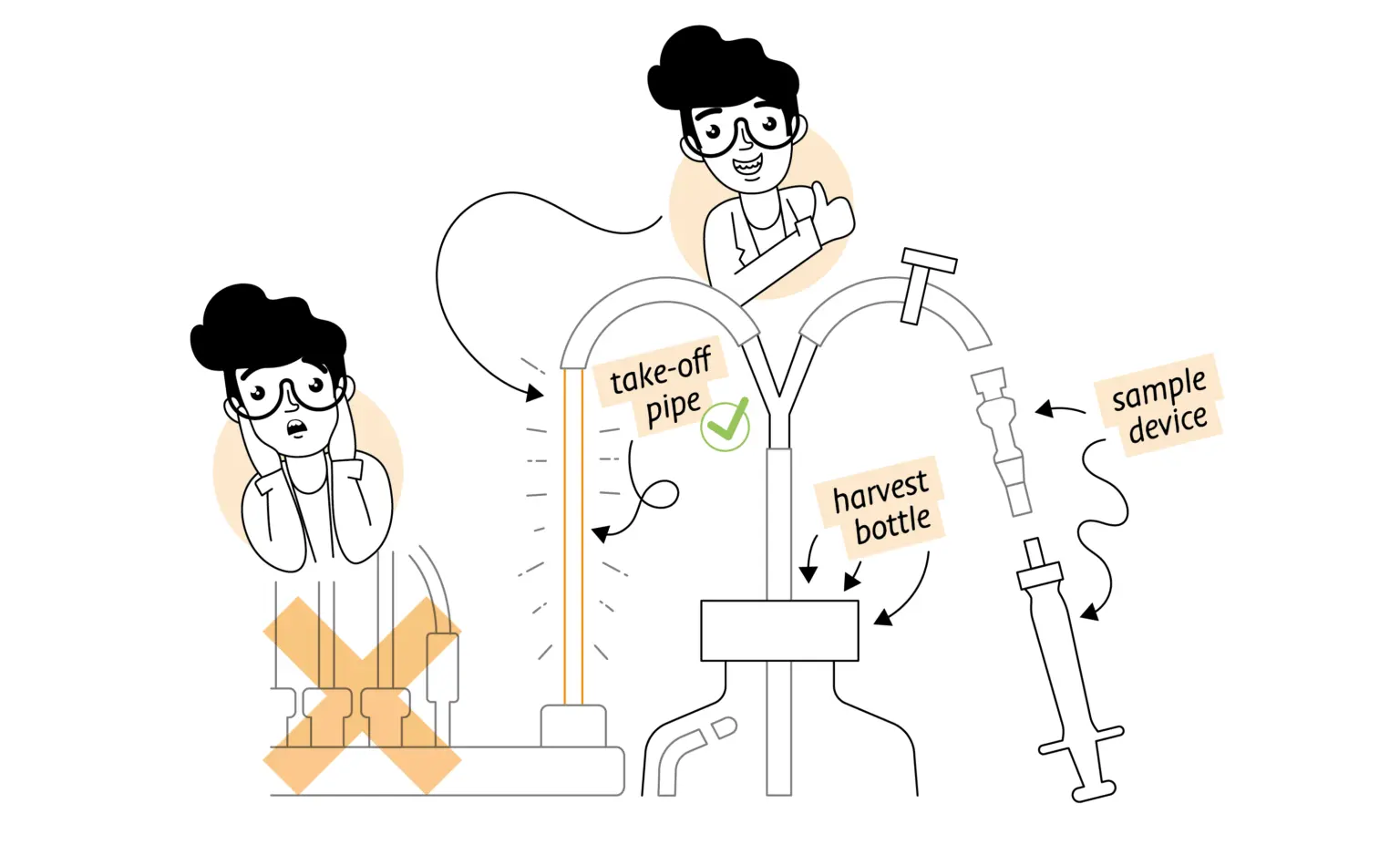
Tip #2: Combine sample and inoculation ports
The need for separate ports for these two essential tasks can be removed for basic applications with a simple Y piece on the sample pipe and a short side arm with a Luer connector. This set up is covered with an aluminium foil cap for autoclaving. The flexible tubing line on the sample pipe is clamped off with a gate clip or Pinchcock Hoffmann. The sample line has its own sample device in place on the other arm other arm of the Y, as normal.
Inoculation relies on using a syringe of suitable size with a Luer end. Syringe volumes up to 60 mL are common and even up to 100 mL. For a small bioreactor of 1 – 2 litres working volume this should be sufficient. The option is to concentrate the inoculum into a smaller volume than usual, so it matches the syringe volume yet delivers the same starting cell concentration in the vessel. Using a single-use septum fitting has the added benefit of being needle-free.
When the inoculum has been added, the side arm with the septum can be clamped off as close to the Y piece as possible. If the used syringe is not an impedance, it can be left in place. If it is, remove it aseptically. When the sample line of the Y is opened. Discarding the first sample drawn up will eliminate the influence of any left-over culture from the inoculation process.
Tip #3: Move OD measurement outside the vessel
Finding a port fitting for an optical density sensor is one of the most common needs for being creative with port allocations. The sensor will need a 13.5 Pg port and these ports are usually taken by pH and pO2 sensors plus an exit gas cooler. One solution is to switch to an external optical density measurement system like the CGQ BioR.
The sensor usually straps onto the outside of the vessel and measures through the glass of the vessel wall. Of course, their use depends on the vessel being accessible for mounting the sensor. Use with a totally enclosed thermal block will be impossible.
Tip #4 for continuous culture: Sample externally from the harvest line
A steady flow of spent culture is being continuously removed from the culture vessel, it can be “tapped into” using a similar set up to the combined sampling and inoculation. A Y piece inserted in the outlet line can have a sample device mounted on it and used in the same way as sampling from the bulk culture in the vessel. This is dependent on the culture being sufficiently homogeneous and the rate of dilution high enough that enough material for a sample is present.
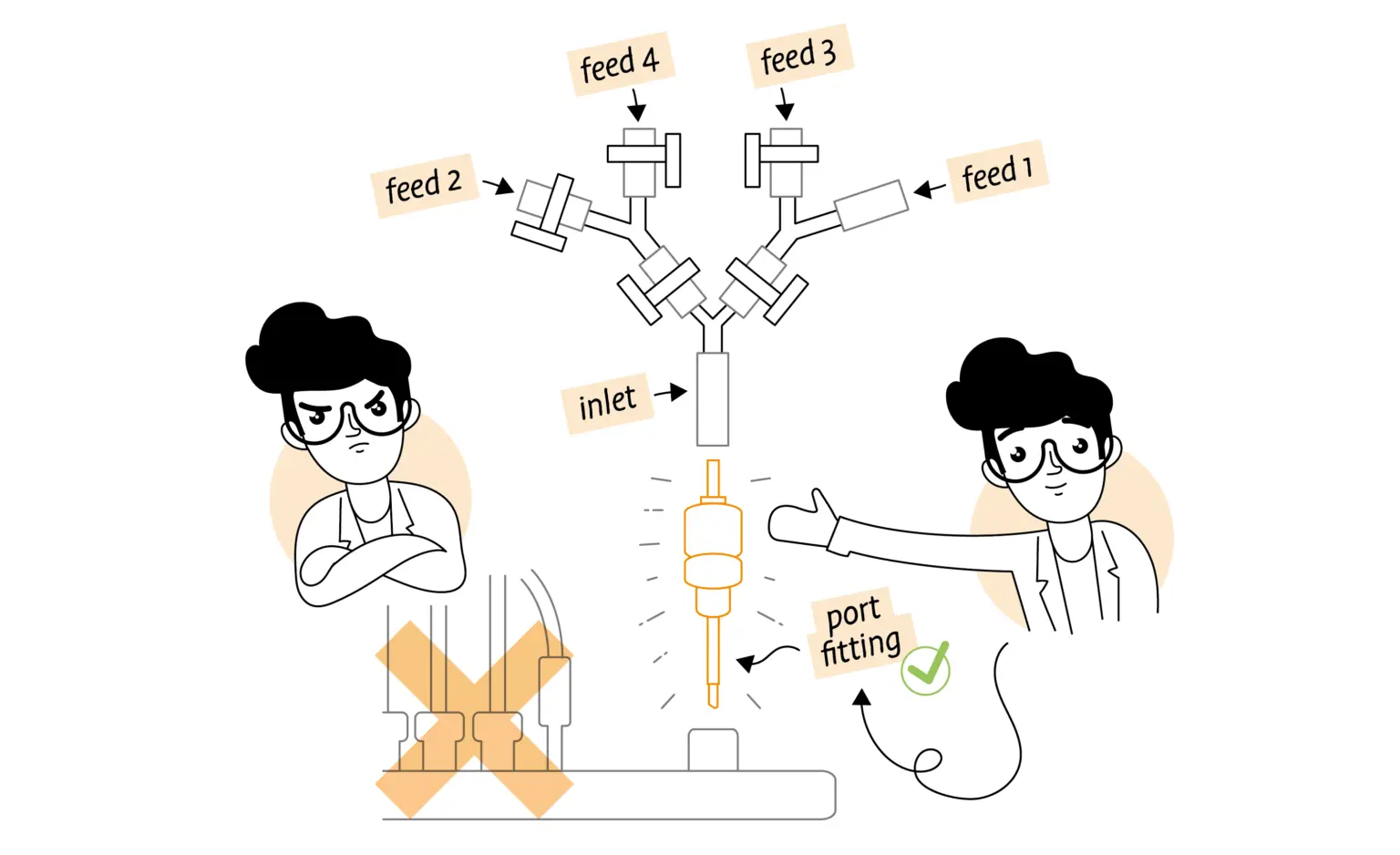
Tip #5: Use the same inlet for different feeds
This is one of the most obvious. Adding one or more Y pieces to a short inlet pipe in the vessel top plate multiplies the number of different feeds which can be added. Examples include adding inductant, changing feedstuff as an inducer and nutrient source and the use of mixed feeds for transitioning phases. It may be that not every feed needs accurate control and setpoint modification. Aseptic connectors again play a part if feed bottles must be exchanged during a running process. If the bioreactor can re-allocate pumps, then both the acid and antifoam pump can be repurposed for additional feeds. Inlet pipes are normally available but of limited diameter for small bioreactors. If so, they may not take account of required flow rates for rapid additions requiring a wider inlet.
Tip #6: Repurpose an unused port with an adaptor
Extending the capability of larger bioreactors depends on the use of 19 mm ports, which are now less common on autoclavable bioreactors. These ports are still used widely on in situ sterilizable vessels but the standard type of port for autoclavable vessels has moved to 13.5Pg. If you have a spare 19 mm port, rather than leave it unused, it might be possible to get an adaptor from the manufacturer to convert it into a 13.5Pg. They are adaptations to the port closure, so the 19mm option always remains for future changes in requirements.