1. Overfilling shake flasks
The usual filling volume for standard Erlenmeyer flasks is between 20% - 40% of total volume e.g. a 250 mL shake flask will contain 100 mL of culture. This is already above the fill volume which produces better oxygen transfer (10-15 %). Specially designed high-density culture flasks are an exception to this general rule. To fill beyond 25 % will both handicap growth of aerobic cultures and can increase wear on shaker bearings by raising the height of the load.
The aim of shaking using an Erlenmeyer flask is to provide good mixing and increase the surface area for oxygen transfer. A smaller volume can be partially distributed over the side wall of the flasks by the shaking action. If the fill volume is too large, a large proportion of the culture will not move from the center of the flask, leaving a deeper liquid level and a smaller surface area for gas transfer.
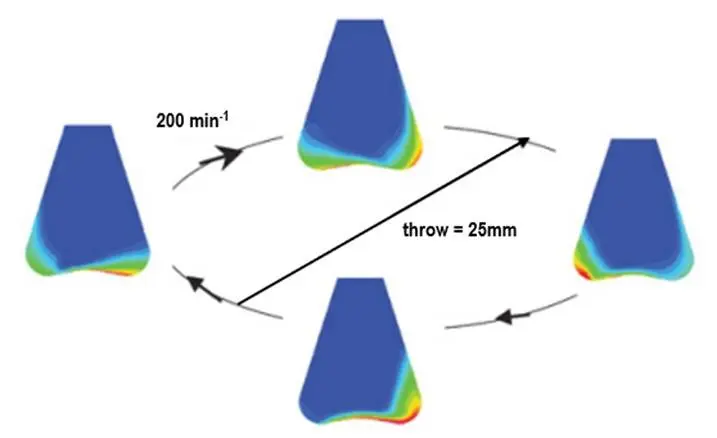
CFD simulation of a Erlenmeyer with 10% filling volume, shaken at 200 min-1. Blue represents 100 % gas phase; red is 100 % liquid phase.
For large flasks, this extra volume also has the effect of raising the height of the load on the shaker platform. This can significantly alter the optimum shaking parameters, as the forces applied varies according to the square of the height i.e. double the height and the maximum weight or speed possible will be reduced to one forth. Avoiding overfilling is better for both the culture growth and the shaking mechanism.
2. Underestimate the need for a cooling system
For any temperature control system to work effectively, there must be a differential between the room temperature and the minimum control temperature. If cooling is not available, the only action for a temperature controller is to turn off the heating if the actual value exceeds the set point.
The effect on the shake flask culture can be dramatic. For mammalian cells, just a single degree overtemperature can be enough to either kill the cells or, at least, severely reduce viability and growth. This makes the absolute minimum temperature for an incubator shaker without cooling approximately 30 °C, if a guaranteed room temperature of 20-22°C can be achieved in all circumstances.
This is already too high for insect cell cultures and borderline for some yeasts. Ambient temperatures of 30°C+ in Summer are almost certain in many parts of the world. Cooling is practically a standard for all but thermophilic cultures unless the laboratory has a very efficient air conditioning system. Cooling just the incubation chamber will be both more efficient and less expensive.
3. Frequent door opening
The need to open the incubation chamber door for sampling, feeding or exchange of flasks has the following effects:
- Temperature control will stop, with fans switching off to help maintain the chamber temperature
- The shaking will stop, with a gentle slow-down taking some seconds
- Access to the flasks is necessary, the door will remain open for seconds or even minutes
- When the operations are finished, the door must be closed, the shaker gently re-starts and the temperature control switches on again
Total up the time when the culture is not at the correct temperature and shaking speed and compare this to the total duration of the culture. For short cultures e.g. 12 hours or less, this can be significant.
This is very unlikely to kill your culture, but it is likely to affect:
- Growth and viability due to sub-optimal mixing and oxygen transfer
- Time to reach a specific biomass level for e.g. adding inductant
- Statistical validity of replicates e.g. do the flasks nearest the chamber door get cooler?
One answer to this problem is to add options to your shake flasks, so that biomass estimation and even simple feeding can be achieved automatically without opening the chamber door. The issue of adding and removing flasks can be due to common usage. If the incubator shaker is used by multiple groups, each one will add and remove flasks at different times. This can lead to a significant amount of time when the cultures are not at the correct temperature and growth suffers for all users. This issue can be solved by booking sole use of an incubator shaker. If this is leading to large units containing just a few flasks, it may be more efficient to consider having more small incubator shakers available.
4. Taping a reference thermometer to the incubation chamber door
Taping a reference thermometer to the clear window of an incubator shaker is not a “sin” in itself, but believing the value certainly is. The key point is that the reading is NOT the temperature of the incubation chamber. It’s an amalgam of the temperature of the air inside the chamber reaching the door and the seepage of ambient temperature through the clear panel.
Believing this reading is accurate can lead to a decision to change the control value by a degree or more to make the thermometer reading match the desired growth temperature. This is simply wrong when you consider the temperature which matters is a centimeter or two above the shaker tray where the culture is located. The temperature sensor used as a reference for control is located in the main air stream and closer to the shaking platform.
For the ultimate accuracy, an optional external temperature sensor mounted in a flask on the shaking platform can be used. This must be filled to a similar volume as the growth flasks and located in a good position to ensure the maximum stability of the reading. However, the greatest benefit will be achieved by just taking the supposed reference thermometer off the chamber window.
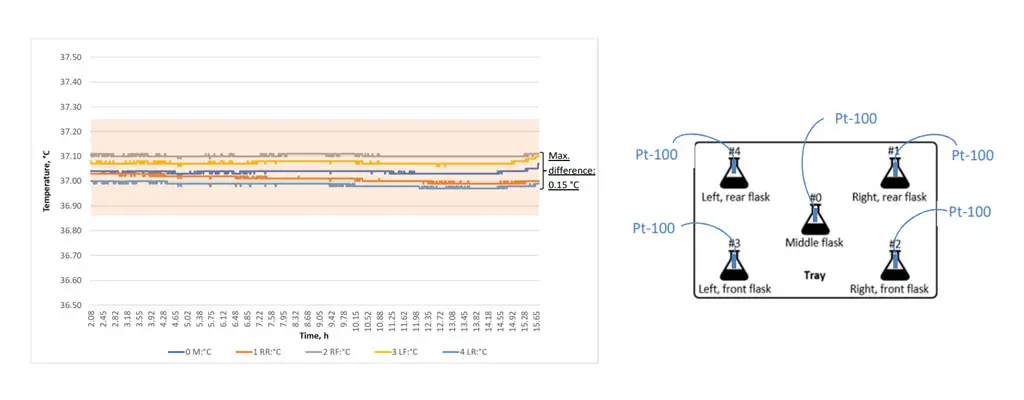
Example data from a temperature uniformity validation showing the uniform temperature control of an INFORS HT Multitron incubator shaker. Right: Excerpt of a protocol for temperature uniformity validation.
5. Pack items around the incubator shaker
This is something that can happen over time with no obvious reason why it could be harmful.
An incubator shaker depends on a good airflow around it on all sides for:
- Drawing fresh, oxygenate air into the incubation chamber
- Removing excessive heat from the chamber
- Cooling of electrical components e.g. a cooling compressor
However, this clearance may be considered as a good place to store boxes, packing, or even further reduced by moving the incubator shaker nearer to a wall. This will result in components running hotter than indented, reduced movement of air through the chamber and a source of clutter interfering with good cleaning.
The answer is simple: Please be guided by the manufacturer’s instructions on clearance and ensure anything finding its way into this space is removed promptly.
6. Not dealing with evaporation losses
As air is exchanged between the head space above the shake flask culture and the main incubation chamber, it is inevitable that some moisture will be lost at temperatures above ambient. For a short culture with large volumes, this may be insignificant. However, if a small culture volume is used with a culture of long duration, changes in the concentration of solutes and osmolarity can interfere with growth. This is especially true for mammalian cell cultures.
Evaporation can result in growth limitations and reduced productivity due to many factors, including:
- Increase in osmolarity
- Increase in concentration of salts and ions
- Changes to oxygen solubility
- Increased viscosity
As an aside, this is even more apparent when micro-well plates are cultured, as the volumes are much lower at the outset. For plates, a box with reduced ventilation may be used to reduce evaporation losses. In some cases, special covers over each plate which allow gas exchange can also help prevent moisture loss.
The “catch all” solution in this case is to specify a humidification system for the incubator shaker. This can use ultrasonics for aerosol creation from a bath inside the chamber, or a small steam generator can directly inject steam. An advantage of the latter system is that it removes a potential place for contaminants to grow i.e. the water bath. For a comprehensive overview on the different humidification systems available on the market have a look at our blog post: Preventing Evaporation: Humidification Systems in the Incubator Shakers.
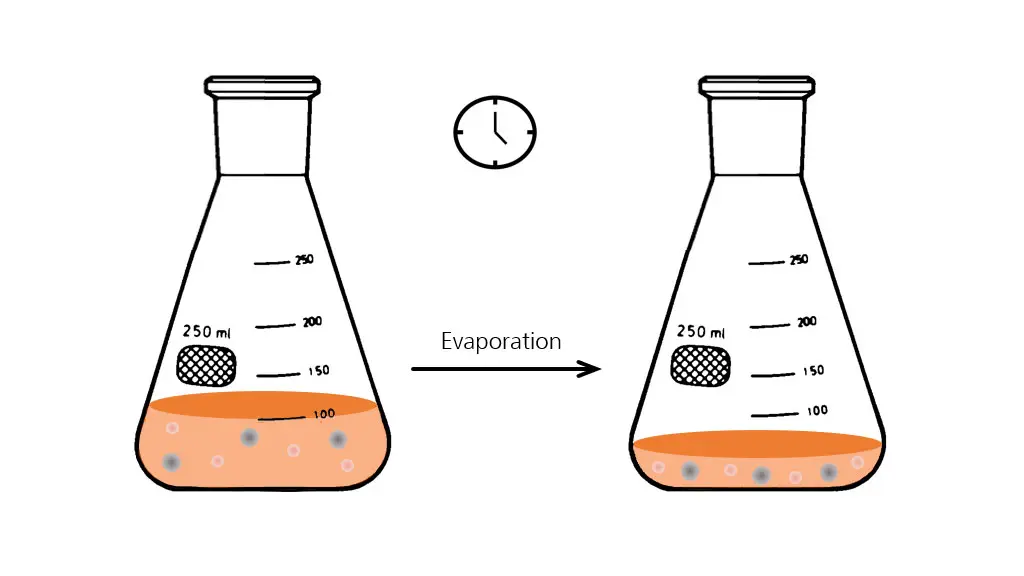
Evaporation in shake flasks: Working temperatures above ambient and long cultivation periods result in an inevitable loss of liquid due to evaporation.
7.Not having a regular cleaning schedule
Contamination of the incubation chamber is a serious risk, which can be hard to remove once it is established. This can be caused by spillages getting below the shaker platform, where they may be hard to reach and clean up. A flask breakage is the most likely cause in this case. However, smaller spills caused by rough handling or transfer on the outside of flasks can lead to problems over time.
Rather than let this situation progress until full disinfection and/or sterilization is required, a prompt reaction to spillages and a regular cleaning schedule can stop the problem before it starts. If the incubator shaker is a shared resource, this could be organized on a rota schedule, so no single operator must do this task very often. Our blog post “How to Clean Your Incubator Shaker” guides you through all the essential cleaning steps.
A best practice routine can be added to any SOPs for general cleaning:
- Cleaning surrounding areas should be designated to a person, group or contractor
- A schedule should be agreed regarding frequency and thoroughness of the cleaning process
- A log should be kept detailing results of earlier untreated spillages
Good design can help! A shaker platform which can be easily moved for access beneath is a real benefit. A smooth chamber base with rounded corners and a drain point will allow for vigorous rinsing out of serious spills. Options such as an anti-microbial coating or UV sterilization of the air stream can then deal with the much lower levels of potential contaminants which can enter from the laboratory environment.
Conclusion: The issues reviewed are all easy to correct!
With some foresight and an appreciation of why they are problematic, avoiding these mistakes is simple. In addition, most solutions are cost-free or inexpensive:
- Only fill flasks to maximum 20 % of total volume.
- Ensure an adequate differential with room temperature for good control or actively cool.
- Minimize door opening during cultivation.
- Don’t use a thermometer taped to the chamber window.
- Keep the area around the incubator shaker clear of obstructions to air flow.
- Be aware of the potential for evaporation losses over time and plan for this.
- Institute a regular cleaning regime and deal with spills right away.
Avoid the “7 deadly sins” and your cultures will be pure and productive.