Batch processes
In a batch process, all nutrients are provided at the beginning of the cultivation, without adding any more in the subsequent bioprocess. During the entire bioprocess, no additional nutrients are added – just control elements such as gases, acids and bases; it is a closed system. The bioprocess then lasts until the nutrients are consumed. This strategy is suitable for rapid experiments such as strain characterization or the optimization of nutrient medium. The disadvantage of this convenient method is that the biomass and product yields are limited. Since the carbon source and/or oxygen transfer are usually the limiting factor, the microorganisms are not in the exponential growth phase for a long time.
To improve the availability of dissolved oxygen, the oxygen transfer rate must be increased. This is achieved by increasing the stirring speed, the gas flow, the proportion of oxygen in the gas mix, or the pressure (if the bioprocess takes place in a steel bioreactor). Since the combination of the various parameters is intended to improve the concentration, sophisticated management and control processes of these parameters are needed. These processes, known as cascades, can be configured individually to adapt them to a specific application. One or more parameters that are supposed to be used for adjusting the concentration of dissolved oxygen are predefined at the controller. The first step towards reaching the target value is to vary the first parameter (for example stirrer speed) within the defined range. If that does not work, the next step is to change subsequent parameters until the target value can be maintained.
After the end of a bioprocess run in batch mode, only the biomass or medium is harvested and appropriately processed to obtain the desired product. From the bioreactor point of view, the process is repeatedly interrupted by cleaning and sterilization steps, and the biomass is only produced in stages. In addition to the low yield of biomass, batch processes have also an increased risk for substrate or product inhibition. The latter describes the interference of enzyme activity by the presence of high concentrations of substrate or product, which might induce metabolic feedback that can drastically reduce the yield.
The advantages of a batch culture are:
- Short duration
- Less chance of contamination as no nutrients are added
- Separation of batch material for traceability
- Easier to manage
Some disadvantages include:
- Product is mixed in with nutrients, reagents, cell debris and toxins
- Shorter productive time
- Can involve storage of batches for downstream processing
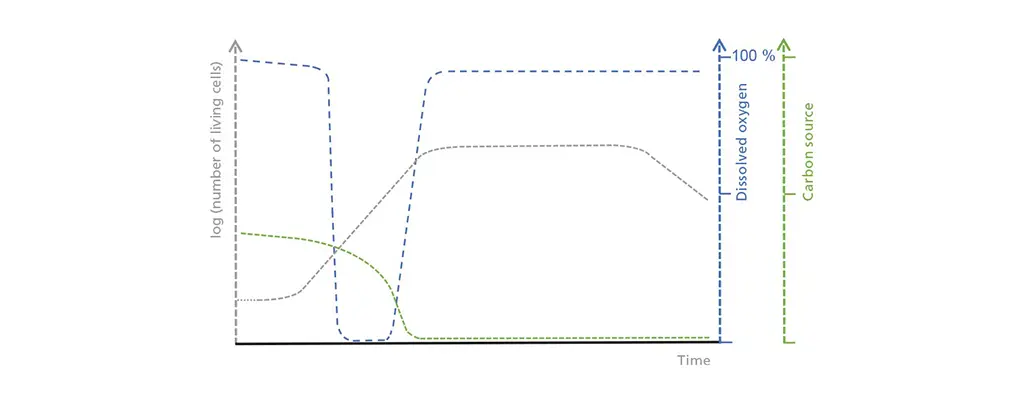
Schematic illustration of the correlations between living cell concentration, dissolved oxygen, and the limiting carbon source in batch operation. In the initial lag phase, the living cell count only increases slowly, which leads to a moderate but steady uptake of the carbon source. Oxygen consumption increases during the exponential growth phase until it exceeds possible oxygen input. Once the carbon source is depleted, the stationary phase starts and is followed by a dead phase, during which the living cell count drastically decreases.
Fed-batch processes
One way of keeping nutrients from becoming a limiting factor is to constantly supply them during cultivation. This is called a fed-batch process, which is a partly open system. The advantage of feeding during cultivation is that it allows to overall achieve higher product quantities overall. Under specific growth conditions, the microorganisms and/or cells constantly double and therefore follow an exponential growth curve. This is why the feed rate should increase exponentially as well. Generally, the substrate is pumped from the supply bottle into the culture vessel through a silicone tube. The user can either manually set the feed at any time (linear, exponential, pulse-wise), or add nutrients when specific conditions are met, such as when a certain biomass concentration is reached or when a nutrient is depleted.
The fed-batch process offers a wide range of control strategies and is also suitable for highly specialized applications. However, it may increase the processing time and potentially leads to inhibition through the accumulation of toxic by-products. The user also needs to have a more in-depth understanding of bioprocesses to do this, which should, however, not be interpreted as a disadvantage.
While the batch process is classified as a discontinuous process, a fed-batch process is a semi-continuous process. During experiments at the beginning of the last century with the aim to produce as much biomass as possible from baker’s yeast in a batch process, excessively high substrate concentrations (in this case glucose) were found to inhibit growth, mainly by the formation of ethanol. On the other hand, this property of baker’s yeast can be used to produce ethanol. At high glucose concentrations and sufficient dissolved oxygen in the medium, alcoholic fermentation still occurs, which is called the Crabtree effect. This effect is used in some food production processes with yeast.
Due to their advantages, fed-batch processes per se are now used in all areas of biotechnological production, in particular for the production of recombinant proteins and antibiotics.
The advantages of a fed-batch culture are:
- Extends a culture’s productive duration
- Can be used to switch genes on or off by changing substrate
- Can be manipulated for maximum productivity using different feeding strategies
Some disadvantages include:
- Allows build up of inhibitory agents and toxins
- Provides another point of ingress for contamination
- May produce high cell density numbers and product yields which are difficult to deal within downstream, creating bottlenecks in the whole process.

Schematic illustration of the relationship between the living cell concentration, dissolved oxygen, and the limiting carbon source in the fed-batch process. When implementing a fed-batch process, you will need to adding the feed immediately after the exponential phase, to prevent the carbon source from being exhausted (thick green line vs. dashed green line). Shown here is an exponential feeding process in which exponentially growing organisms remain in a prolonged exponential phase (thick gray line vs. dashed gray line). This also means that the quantity of consumed oxygen increases, which is why the amount of dissolved oxygen in the medium is lower (thick blue line vs. dashed blue line). In this example, a growth rate μ less than μmax has been selected for the feeding phase.
Continuous culture
After a batch growth phase, an equilibrium is established with respect to a particular component (also called steady state). Under these conditions, as much fresh culture medium is added, as it is removed (chemostat). These bioprocesses are referred to as continuous cultures, and are particularly suitable when an excess of nutrients would result in inhibition due to e.g. toxin build up or excessive heating. Other advantages of this method include reduced product inhibition and an improved space-time yield. When medium is removed, cells are harvested, which is why the inflow and outflow rates must be less than the doubling time of the microorganisms. Alternatively, the cells can be retained in a wide variety of ways (for example, in a spin filter), which is called perfusion. In a continuous process, the space-time yield of the bioreactor can be even further improved compared to that of a fed-batch process. However, the long cultivation period also increases the risk of contamination and long-term changes in the cultures. Moreover, continuous processes are ideal tools for gaining a better understanding of the process, since all process parameters remain constant when the system is operating correctly.
The three most common types of continuous culture are:
- Chemostat: The rate of addition of a single growth-limiting substrate controls cell multiplication.
- Turbidostat: An indirect measurement of cell numbers (turbidity or optical density) controls addition and removal of liquid. This needs an additional sensor but is driven by real-time feedback.
- Perfusion: This type of continuous bioprocessing mode is based on either retaining the cells in the bioreactor or recycling the cells back to the bioreactor. Fresh medium is provided and cell-free supernatant gets removed at the same rate.
The advantages of a continuous culture are:
- Allows the maximum productivity
- Time for cleaning, sterilization and handling of the vessel are all reduced
- Provides a steady state for metabolic studies when many elements sum to zero
Some disadvantages include:
- Difficult to keep a constant population density over prolonged periods
- The products of a continuous process cannot be neatly separated into batches for traceability
- Increased risk of contamination and/or genetic changes
Repeated Fed-batch / Semi-continuous Culture
Besides fed-batch or continuous culture, there are hybrid methods that can be useful when running a bioprocess. For example, harvesting all but a small residue of a completed (fed) batch and leaving the remaining cells available to use as an inoculum for the next filling of the vessel. This feeding strategy bridges the gap between fed-batch and continuous methods and solves key issues related to using continuous culture in pharmaceutical and other production environments. This application applies to Single Cell Protein (SCP) production, as well as fermentative production of lipids, fatty acids, and penicillin.
It is a straightforward concept: after a period of batch culture, a significant part (between 25 to 75%) of the bioreactor working volume is removed and replaced with fresh media, including the carbon substrate. The remaining suspension culture in the bioreactor acts as an inoculum for the next batch. This process is repeated over several cycles, the time period being determined by the specific growth rate and substrate utilization profile. Typically, after several days, the process is terminated.
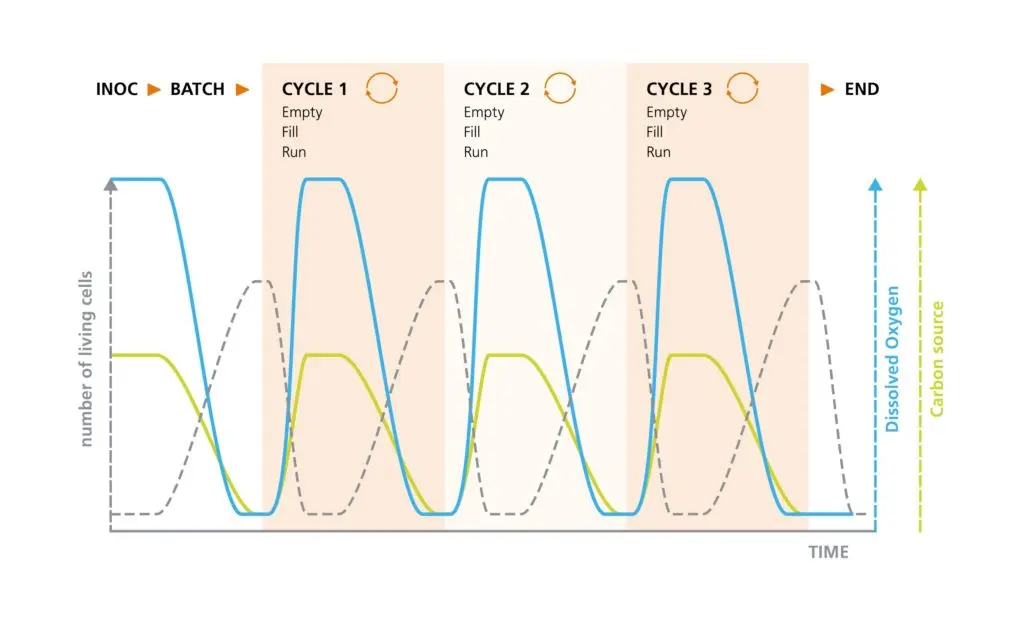
Schematic illustration of the correlations between living cell concentration, dissolved oxygen, and the limiting carbon source in a repeated Fed-batch operation. At the end of batch cultivation, between one quarter and three quarters is harvested. The existing culture is used as inoculum for the next cycle and is supplemented with a fresh culture medium. Once the carbon source is exhausted, a new cycle can be started again by partial emptying and filling. The number of cycles is determined by the required biomass.
In a fed-batch, the feed rate is decisive to actively control the growth. In contrast, almost all repeated processes aim at substrate accumulation, which means no active influence on growth is possible. As a suitable analogy, the process can also be described as a repeated batch. Thus, different approaches with corresponding terminologies can also be found in the literature. “Semi-continuous culture” is another alternative description.
This methodology has the following advantages over fed-batch:
- Potential accumulation of toxins and unwanted metabolites is prevented due to the medium exchange
- Culture density will not reach a point where issues arise due to an increase in biomass such as the oxygen transfer or cooling capacity.
- Yields of biomass and proteins remain constant across cycles.
The benefits compared to full continuous culture are as follows:
- The culture effluent is less diluted, reducing downstream processing requirements.
- Allows for segregation of product into sub-batches categorized by time, which has a positive impact on quality control and troubleshooting.
- The overall length of repeated fed-batch cultures is closer to fed-batch i.e. several days, rather than weeks or months.
Summary and bioreactor recommendations
Modern stirred tank bioreactors give you full flexibility to run batch, fed-batch, continuous, or hybrid processes depending on your goals. With automated feeding, dissolved oxygen control, and integrated software platforms like eve®, you can design and optimize your bioprocess at any scale.
Quick Guide: